Introduction
Sure, here is an introduction about the difference between work and heat:
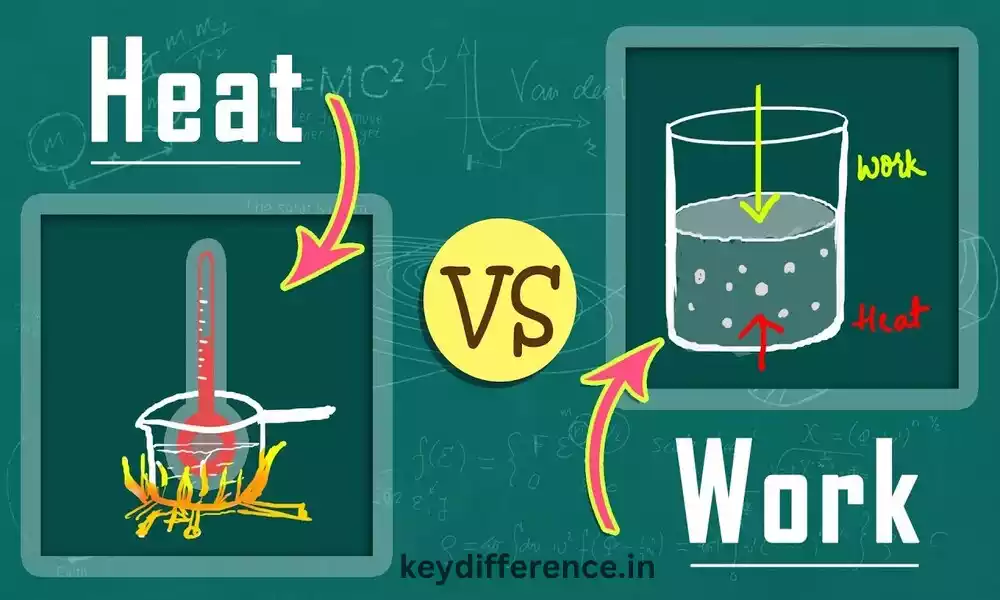
Work and heat are both forms of energy, yet their transfer mechanisms differ considerably. Work involves moving energy from one system to the next by applying force; heat involves exchanging it via temperature differences between systems.
Work can be accomplished in various ways, including lifting an object, moving it or altering its shape. Heat can be transferred in three ways: conduction, convection and radiation.
Conduction refers to the transference of heat through direct contact. For example, when you touch a hot stove with your hand, conduction occurs and heat from it transfers directly onto your hand through conduction.
Convection is the transfer of heat through movement of fluid. For instance, when boiling water on your stovetop, its heat is transferred via convection into the pot’s contents via convection – hotter water rises while cooler waters settle to the bottom, helping spread out evenly distributed heating across its entirety.
Radiation is the transference of heat through electromagnetic waves. For instance, when standing next to a fire, heat from it is transmitted directly onto you through radiation. Fire emits electromagnetic waves carrying energy that strike against your skin and heat it up as their energy is transferred onto it by contact.
Heat transference between systems depends directly on their temperature difference; the bigger this gap is, the faster heat will flow between systems.
Work and heat can be converted into each other; for instance, when you use a stove to prepare food, its heating element converts its heat into work by turning electrical energy from outlets into heat that then gets transferred onto food.
Defining work
Work, in physics, is the transference of energy from one system to another through force application. Work occurs whenever an object is moved a certain distance and equals to the force applied times distance travelled. The amount of work accomplished equals force times distance traveled.
Formula for work: W = F * d The formula for work can be written as W = F * d, where W is work done in joules and F is force in newtons (the equivalent units are WJ = work completed per second and F = force applied per newton). Please be wary when writing code with this equation! For your safety. Learn more .
Work can either be positive, negative, or zero; work being positive when force and displacement move in the same direction while negative when they act in opposing directions and zero when no displacement exists.
Work is an energy form. When work is completed, energy transfers between systems; those who do the work will lose energy while those receiving it gain energy.
Work is a fundamental concept in physics that describes the movement and transference of energy between objects.
Calculation of work
Calculating work depends on both the circumstances and type of force involved, so here are formulas for calculating it in some common scenarios:
Work (W) can be measured using constant force in one direction of motion: W = Force (F) x Displacement (d) x Cost, where W is measured in either Joules (J) or foot-pounds (ft-lb).
F represents the magnitude of constant force applied, measured in newtons (N) or pounds (lb). D represents object displacement measured in meters or feet (ft).
The represents the angle between force vector and displacement vector; costh equals 1 since force and displacement are aligned. Work performed by variable forces:
When an object experiences non-constant force, in order to calculate work you must integrate this force with respect to displacement: W = F * ds, where W is measured in either joules (J) or foot-pounds (ft-lb).
F is the force vector at each point along the path; ds represents an infinitesimally small displacement vector. Work Done against Gravity: To calculate work done against gravity when lifting something against it, work can be calculated as follows:W = F*H for every unit of work completed against gravity
W stands for work, measured in either joules (J) or foot-pounds (ft-lb). F stands for the force against gravity applied to an object by gravity itself; typically its weight, measured either as newtons (N) or pounds (lb). Finally, h represents its vertical height or displacement from its location on Earth’s surface.
Consideration should be given to selecting an appropriate formula and units based on your situation and type of force involved.
Work formula
Calculating work depends on both the situation and type of force involved, but here are the main formulas used to do so in different scenarios:
Work done by constant force acting in one direction of motion: W = F * Displacement (d) * Cost W represents work measured in either Joules (J) or Foot-pounds (ft-lb).
F is the magnitude of constant force applied, measured in Newtons (N) or pounds (lb). D represents object displacement measured in meters or feet.
At its root, th represents the angle between force vector and displacement vector, in this case costh is used to account for force components in the direction of motion. A variable force:
If the force acting on an object is not constant, to calculate its work you must integrate its force with respect to displacement: W = F * ds Work (W) represents work measured in either joules (J) or foot-pounds (ft-lb).
At each location along the line, F stands for the force vector, and ds for a very tiny displacement vector. Work Done against Gravity: To calculate work done against gravity when lifting something up against its own weight, work is calculated as follows: W = Fx H, with “h” equalling Height in meters.
W stands for work, measured in either joules (J) or foot-pounds (ft-lb).
F stands for force applied against gravity – typically, weight of object being supported – measured either as newtons (N) or pounds (lb). H represents the vertical height or displacement of an object.
It’s essential to choose an appropriate formula based on your specific circumstances and characteristics of force involved, with units used consistent throughout to ensure accurate calculations.
Units of work
Work units are typically expressed in either joule (J) or foot-pounds (ft-lb). Joule (J) is the standard unit of work in the International System of Units (SI). It can be defined as the amount of energy expended when applying the force of one newton on an object and moving it one meter in its direction.
Foot-pound (ft-lb) is an often used unit of work measurement in imperial and U.S. customary systems of measurement, where one pound of force applied to an object causes it to move one foot in its direction. For conversion between joules and foot-pounds, use this conversion factor: 1 foot-pound (ft-lb) equals 1.355882 joules (J).
Utilize an appropriate unit of work based on your chosen system of measurement to maintain uniformity with calculations or comparisons using other units.
Define heat
Heat transfer refers to the transference of thermal energy between systems due to differences in temperature. Heat can be thought of as energy; however, unlike work it does not transfer through physical exertion but instead occurs naturally over time.
There are three primary methods of heat transfer:
conduction, convection and radiation. Conduction refers to the transference of heat via direct contact. For instance, when you touch a hot stove with your hand, its heat transfers directly onto it through conduction and onto your hand through conduction.
Convection is the transfer of heat through fluid motion. For instance, when you boil water on your stovetop, convection transfers heat directly into it through rising and falling layers of hot and cooler water in your pot, which helps distribute heat more evenly around its interior surface.
Radiation is the transference of heat through electromagnetic waves. For instance, when you stand next to a fire, its heat transfers directly onto you through radiation emitted by its electromagnetic waves carrying energy; once these waves hit your skin they transfer their power and cause it to heat up further.
Heat transfer rates depend on the temperature difference between systems; as this metric increases, so will its rate. As temperatures diverge more quickly, more heat will transfer more quickly between them.
Heat transfer mechanisms
There are three basic ways to transmit heat: conduction, convection, and radiation. Each mechanism possesses unique qualities and occurs in different scenarios; here is an overview of each heat transfer mechanism:
Conduction:
Conductive heat transfer occurs through direct molecular contact between particles within a solid or between solids in contact, such as between solids.
Heat energy flows from higher temperature regions to regions with lower temperatures as particles with greater thermal energy transfer their thermal energy to adjacent particles with lower energy, leading to an overall equalization of temperatures.
Conduction is particularly effective with materials having high thermal conductivity such as metals. Some examples of conduction could include heating up one end of a metal rod slowly and gradually transferring it along its length or touching hot surfaces like touching a hot pan with one touch, then feeling its heat on another side.
Convection:
Convection is the transfer of heat through fluid movement caused by temperature differences. Convection entails moving large volumes of fluid in order to transport heat energy from one location to the next. Natural and forced convection are the two types of convection.
Natural Convection: Occurs when fluid motion is driven by density differences due to temperature variations, with hot fluids tending to rise while cooler ones sink, creating natural circulation loops such as hot air rising from heated surfaces or water boiling in a pot.
Forced convection: Forced convection involves using external forces, such as fans or pumps, to generate fluid motion and enhance heat transfer. Examples of forced-convection heating systems or engine cooling fluid flow could include air circulation systems with forced convection heating or coolant in car engines.
Radiation: Radiation is the transfer of heat using electromagnetic waves. Unlike conduction and convection, radiation does not rely on direct contact between objects for its transference; heat energy can instead travel in waves through vacuum or transparent media to travel further away.
All objects with temperatures above absolute zero emit thermal radiation which varies in intensity according to temperature and surface properties; an example would include feeling warm sunlight on your skin, fire emissions, or infrared heating in kitchen appliances as examples of radiation transference.
Real-world heat transfer occurs via multiple mechanisms. Understanding these is critical in multiple fields – engineering, physics, meteorology as well as everyday applications like heating/cooling systems, cooking and thermal insulation.
Conduction
Conduction is the process of heat transfer that involves direct molecular contact and physical interactions among particles within a substance, where heat energy flows from regions with higher temperature to regions of lower temperature within or between solids in contact.
Particles possessing higher thermal energy (higher kinetic energy) transfer their energy to neighbors with lower thermal energy for an overall equalization in temperature.
Key Considerations About Conduction:
Mechanism: Conduction is the transference of heat energy through vibration and collision of particles within a substance, such as when energy-rich particles vibrate more vigorously to collide with nearby ones and transfer thermal energy.
Temperature Gradient: Conduction occurs when there is a difference in temperature within or between solids in contact, leading to heat transfer from higher-temperature region to lower-temperature region until thermal equilibrium has been achieved.
Material Conductivity: Different materials have different capacities for conducting heat. Metals such as copper and aluminum offer higher thermal conductivities that allow the transfer of heat more rapidly while materials with lower thermal conductivity such as wood and plastic impede transference of heat more readily.
Conduction Equation: Fourier’s Law of Heat Conduction can be used to describe the rate of heat conduction through solids: Q = -kA(dt/dx), where Q is the rate of heat transfer in Watts or BTU per unit time.
Thermal Conductivity = Thermal Conductivity per Meter-Kelvin or BTU per Hour-Foot Fahrenheit). Axis length measures the cross sectional area through which heat transfers, measured either in meters or square feet.(dt/dx) measures temperature gradient (i.e. change in temperature per unit distance in Kelvin or Fahrenheit per foot).
Applications: Conduction has numerous practical uses. Examples of its application include cooking on a stovetop, where heat transfers from the burner to cooking utensil via conduction; heat transfer in building materials where insulation materials help minimize conduction; and electronic devices employing heat sinks as heat dissipaters to dissipate through conduction.
Understanding conduction is vitally important in fields like thermodynamics, material science and heat transfer engineering; it explains the behavior of heat flow while providing tools for designing efficient thermal management systems.
Convection
Convection is a mode of heat transfer characterized by the movement of fluid (liquid or gas), caused by temperature differences within a fluid body which causes densification changes and subsequent fluid movement.
Convection plays an essential part in many natural and engineered systems where fluids play an integral role such as natural hydrological systems or engineered thermal environments.
Here are a few key points about convection:
Mechanism: Convection is the transference of heat by moving fluid particles through space. As temperatures heat up, fluid density decreases, leading to it rising while cooler denser fluid sinks; creating what are known as convection cells or currents – moving heat energy between hotter regions to cooler ones via fluid movement.
Types of Convection: Natural (also referred to as free convection) occurs when fluid motion is caused solely by temperature-driven density variations.
For instance, when heating water, hotter areas at the bottom expand, become less dense, and rise towards the top, while cooler water at the top sinks towards the bottom – helping with heat transfer by acting like natural circulation.
Forced Convection: Forced convection utilizes external forces to create and enhance fluid motion. Typically this is accomplished using fans, pumps, or other mechanical devices; examples include forced air heating systems, air conditioning units with fans installed for cooling electronic components using forced convection, or cooling electronic components using fans for forced cooling of electronic components using forced convection. Forced convection has the ability to increase heat transfer rates significantly compared with natural convection.
Convection Heat Transfer Coefficient (h): The rate of heat transfer through convection can be greatly affected by its convection heat transfer coefficient (h), which measures its effectiveness between fluids and solid surfaces. It depends on factors like fluid properties, flow velocity, surface area and characteristics – with higher values representing more efficient transfer.
Applications: Convection can be observed in various natural and engineered systems, including those like these:
Atmospheric Convection: The movement and formation of weather patterns by air mass movement. Heating and Cooling Systems: HVAC (Heating Ventilation Air Conditioning) systems employ forced convection in order to distribute warm or cool air throughout a space.
Cooking: Convection ovens utilize hot air circulation to evenly and efficiently cook food.
Thermal Management in Electronics: Fans or liquid cooling systems may be used to enhance heat transfer between components to increase performance while preventing overheating of electronic parts.
Convection is essential in fields like fluid dynamics, thermodynamics and heat transfer engineering. Understanding convection helps engineers design efficient cooling systems while understanding weather patterns as well as optimizing heat transfer in various applications.
Radiation
Radiation is a method of heat transfer using electromagnetic waves. Unlike conduction and convection, radiation does not require direct contact between objects – it can occur either through vacuum or transparent media.
Radiation serves as the primary way to move heat between Sun and Earth as well as being crucial in various natural and artificial systems.
Here are a few key facts about radiation:
Radiation involves emitting, transmitting and absorbing electromagnetic waves. Heat energy is released in the form of electromagnetic waves which travel throughout space and other media – these waves contain energy which may be absorbed by objects they encounter along their journey.
Electromagnetic Spectrum: Electromagnetic waves associated with heat radiation cover an expansive spectrum. This spectrum comprises radio waves, microwaves, infrared radiation (IR), visible light wavelengths, UV radiation levels, X-rays and gamma rays; most heat radiation occurs in the infrared portion.
Emissivity and Absorptivity: Everything heated above absolute zero emits thermal radiation, with its intensity depending on temperature and surface properties of objects involved. An object’s ability to emit and absorb radiation is determined by its emissivity and absorptivity values – typically, dark surfaces have higher values, while light ones typically exhibit lower ones.
Stefan-Boltzmann Law: An object’s rate of emitting thermal radiation is determined by the Stefan-Boltzmann Law, according to which its power (P) radiated is directly proportional to its absolute temperature (T), as expressed as follows: P = esAT4
where P represents its power in watts.
Emitsivity (a dimensionless value between 0 and 1) of an object. Asociati S is the Stefan-Boltzmann constant (approximately equal to 5.67x 10-8 W/(m2*K4)) whilst A is its surface area in square meters and T is its absolute temperature in Kelvin (in Kelvin).
Applications: Radiation can be found both naturally and artificially.
Some examples include: Solar radiation provides heat and light for Earth. Heating and cooling systems that use radiators emit thermal radiation into warm spaces for heating or cooling.
Infrared (IR) cameras and sensors detect and measure thermal radiation for applications including temperature monitoring, night vision surveillance, medical imaging and cooking appliances that utilize infrared radiation for heating or grilling purposes.
Understanding radiation is critical in fields as diverse as thermodynamics, astrophysics, atmospheric science and engineering. It allows engineers to design effective heat transfer systems; assess energy balance across environments; and utilize radiation for technological applications.
Comparison Table of Work and Heat
Heat is used for various applications. For instance, heat can help:
Heating food, homes and metals – as well as powering engines – with heat is one of the central concepts in physics, as it describes energy transfer from one system to the next.
Here’s a comparison table highlighting the main differences between work and heat:
| Aspect | Work | Heat |
|---|---|---|
| Definition | Transfer of energy by applying a force and causing displacement | Transfer of energy due to temperature difference and thermal energy flow |
| Nature of Energy | Transfer of mechanical energy | Transfer of thermal energy |
| Mechanism | Force applied on an object | Temperature difference and thermal energy flow |
| Direction of Energy | Follows the direction of the applied force | From higher temperature region to lower temperature region |
| Effect on Objects | Changes the object’s position or shape | Changes the object’s temperature or phase |
| Conversion | Work can be converted into heat | Heat can be converted into work (in heat engines) |
| Measurement | Measured in joules (or foot-pounds) | Measured in joules (or calories) |
| Symbol | W | Q |
| Vector/Scalar | Scalar | Scalar |
This table summarizes the key distinctions between work and heat. While work involves the transfer of mechanical energy through the application of force and displacement, heat is associated with the transfer of thermal energy due to temperature differences and thermal energy flow.
The direction of energy transfer, effect on objects, and conversion capabilities also differ between the two.
Work is measured in joules or foot-pounds, while heat is measured in joules or calories. Additionally, work is represented by the symbol “W,” whereas heat is represented by “Q.” Both work and heat are scalar quantities.
Work transfers energy in the direction of the force applied
Work involves the transference of energy in response to external forces applied on an object. When work is performed on something, energy is transferred directly into it resulting in either its energy state changing or movement altering as a result of doing it.
Physics defines work as the product of force applied to an object and its subsequent displacement in response to that force, with mathematicians often denoting this process with W as its symbol.
W = F * d * costh
This formula represents work as measured in either joules (J) or foot-pounds (ft-lb), while F is the magnitude of force applied to an object, measured either in newtons (N) or pounds (lb).
D represents displacement along the direction of force, measured in meters or feet (ft). T represents the angle between force vector and displacement vector; Cth stands for component of force acting against displacement;
If the force and displacement vectors are aligned (th = 0deg), then the costh term becomes 1 and the work formula can be simplified:
W = F * d
This equation states that when forces and displacement vectors are aligned, all forces contribute equally towards accomplishing work on an object.
Simply stated, work involves the transference of energy in the direction of an applied force; its magnitude varies with both force applied and displacement in its path.
Heat can change the object’s temperature or phase
Heat transfer can result in changes to an object’s temperature or phase, producing significant shifts.
Heat transfers between objects cause their internal energy levels to increase and, as a result, temperatures rise. Heat Energy Absorbed by an object Increases its particles’ kinetic energy and causes them to move more Vigorously, Contributing to an increase in Temperature.
Quantifying the relationship between heat transfer and temperature changes can be done using the specific heat capacity of material. The specific heat Capacity measures the amount of heat needed to raise the Temperature of a given mass by an Amount of heat; these vary between Materials Depending on their specific heat capacity; this Determines how Effectively they absorb and store heat.
Heat transfer can alter more than just temperatures; it can also transform substances’ phases by providing or withholding enough heat. As more or less heat is added or taken away from substances, they may undergo phase transition between solid, liquid, and gaseous states.
As heat is applied to solid objects, they can transform from their solid state into liquid form – known as melting or fusion. Conversely, when heat is removed from liquids they solidify and transition back into solid form – known as freezing.
Furthermore, adding heat can vaporize them and turn into gaseous state (known as evaporation or boiling), while when taken away again can condense and change into a liquid state again – giving us two opposite processes at work here.
Specific heat capacity and latent heat (the amount of energy necessary for phase transitions) are two properties that accurately describe how heat affects temperature and phase changes of a substance.
Heat transfer can alter an object’s temperature by either increasing or decreasing, and also can induce phase transitions between solid, liquid, and gaseous states.
Effect on objects
Heat transfer has different impacts on objects depending on factors like its intensity and duration, properties of the object itself, and any specialized heat transfer mechanisms involved. Here are some effects of heat transfer on objects:
Temperature Change: Heat transfer can result in significant temperature variations for an object. As heat is added, its temperature typically increases; when removed, its temperature usually decreases; this variation can have significant ramifications on an object’s properties, functionality and behavior.
Expansion and Contraction: Many materials expand when heated and contract when cooled, leading to significant dimensional changes. When applying measurements or tight fits or structural integrity are key considerations, expansion/contraction effects must be carefully considered in applications where precision, tight fit or structural integrity is crucial.
Structural Alterations: Heat transfer can result in dramatic structural modifications for objects made from materials with low melting points or weak structural integrity, including deformation, melting, warping or disintegration of an object compromising functionality or even leading to damage. Excessive exposure can even cause disintegration causing serious functional impairments or harm.
Phase Transitions: Heat transfer can trigger phase transitions in substances, transforming their states from solid, liquid and gaseous. These shifts have an impact on physical properties and behavior of objects; for instance, freezing or solidification of liquids may result in crystal formation or modification in material strength.
Chemical Reactions: High temperatures can trigger chemical reactions within objects, leading to chemical transformations, decomposition, or combustion that alter their composition, structure or properties – potentially having either positive or negative outcomes depending on how it’s used.
These chemical changes could include changes to composition, structure or properties, decomposition or combustion that alter properties such as composition or structure – as well as decomposition that releases carbon emissions that contributes to global warming.
Thermal Stress: Uneven or rapid heating/cooling can create thermal strain in an object. Expansion/contraction differences among various parts may cause internal stresses that lead to cracking, fracture or mechanical failure of said object.
Material Degradation: Prolonged exposure to high temperatures or repeated thermal cycling can erode certain materials over time, reducing strength, altering electrical conductivity and decreasing elasticity – among other undesirable side-effects.
Understanding the effects of heat transfer on objects is crucial for numerous applications, including thermal management, material selection, design considerations and safety precautions. Understanding heat transfer enables appropriate handling, protection and optimization of objects subjected to these processes.