Introduction
Chemical reactions play a vital role in synthesizing complex molecules and understanding their behavior. Oxymercuration and demercuration are two distinct processes involving organomercury compounds interacting with unsaturated carbon-carbon bonds to form complex organic molecules, with functional groups introduced selectively or removed selectively through organomercury compounds.
Both processes hold great significance in synthetic chemistry allowing chemists to introduce functional groups into organic molecules or remove mercury moieties selectively from them.
This content outline seeks to explore and compare the differences between oxymercuration and demercuration. It will explore their core aspects such as definitions, reaction mechanisms, stereochemistry, regioselectivity and applications for each process.
Understanding these distinctions allows chemists to design efficient and selective synthetic strategies with long-term benefits for various fields such as drug discovery, materials science or the creation of novel chemical methodologies.
Let’s now dive deeper into each reaction, exploring their unique characteristics and applications.
What is oxymercuration?
Oxymercuration is a chemical process in which water is added to alkenes or alkynes by using mercuric salt as a catalyst, producing cyclic intermediates known as mercurinium ions which are later attacked by nucleophiles such as water.
Oxymercuration reactions are frequently employed in organic synthesis to convert alkenes or alkynes into alcohols with more hydrogen attached – markovnikov alcohols; their nucleophile is preferentially attacked by nucleophiles such as water; its selective nature makes oxymercuration an invaluable asset in organic chemistry, providing controlled addition of water onto unsaturated compounds.
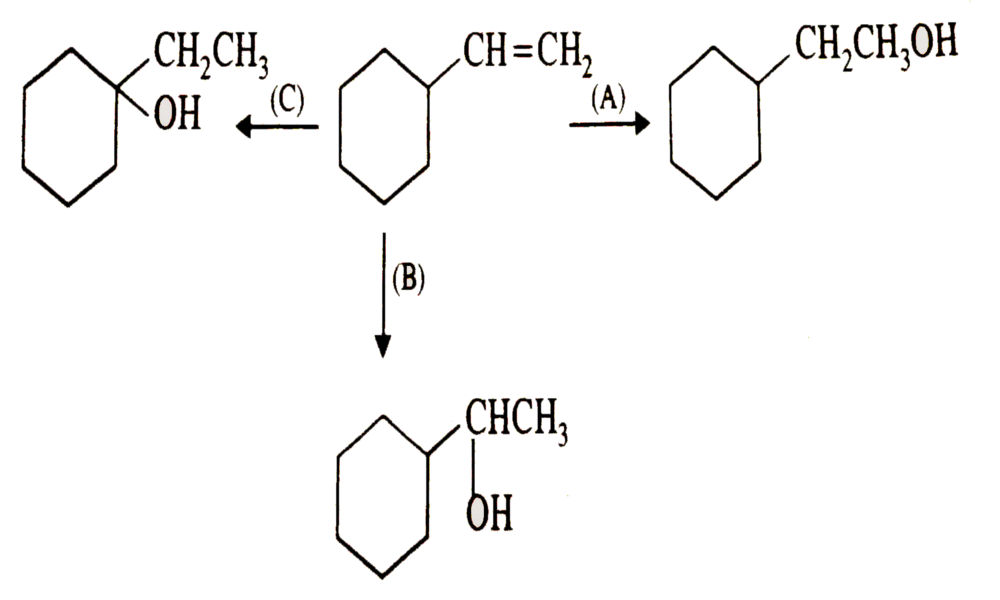
Reaction mechanism
Reaction mechanisms for the conversion of alkenes to alcohols may differ depending on which approach is taken, with acid-catalyzed hydration and oxymercuration-demercuration two common approaches being explored here.
Acid-Catalyzed Hydration Mechanism:
Step 1: Protonation of the Double Bond To initiate this step, an alkene reacts with an acid catalyst such as sulfuric or phosphoric acid to form a carbocation intermediate. In this step, one or both carbon atoms of its double bond receives one proton from the acid catalyst in order to initiate protonation; it could happen either way along the double bond.
Step 2: Nucleophilic Attack by Water
Water acts as a nucleophile by attacking positively charged carbon atoms of carbocations molecules with positive charges on them, which ultimately forms bonds between these carbon atoms and oxygen atoms of water – leading to protonated alcohol intermediates with positive charges on oxygen atoms.
Step 3: Deprotonation
The protonated alcohol intermediate will undergo deprotonation, typically through exposure to water or an acid. Deprotonation involves extracting one proton from each positively charged oxygen atom and thus creating the final alcohol product.
Acid-catalyzed hydration involves protonating an alkene to form a carbocation, followed by nucleophilic attack from water molecules and then subsequent deprotonation into alcohol products.
Oxymercuration-Demercuration Mechanism:
Step 1: Oxymercuration During the oxymercuration step, alkene reacts with a mercuric salt such as mercuric acetate (Hg(OAc)2) in the presence of water or alcohol as solvent. The mercuric salt coordinates with double bond sites on alkene molecules to produce cyclic mercurinium ion intermediates containing one carbon from a double bond while developing positive charges on another carbon of said double bond chain.
Step 2: Demercuration
To demercurate, an acid such as sodium borohydride (NaBH4) or sodium cyanoborohydride (NaBH3CN) must be added to the reaction mixture in order to selectively reduce mercury atoms until their removal and substitution with hydrogen atoms leads to formation of alcohol products.
The oxymercuration-demercuration mechanism works through two steps, with an intermediate form mercurinium ion created during oxymercuration, followed by selective reduction of mercury atoms during demercuration.
These mechanisms provide a general outline of the steps involved in the conversion of alkenes to alcohols using acid-catalyzed hydration and oxymercuration-demercuration methods. However, please keep in mind that actual mechanisms may involve additional steps, intermediates, or side reactions depending on substrate specific conditions and substrate composition.
Stereochemistry of oxymercuration
Oxymercuration refers to the stereochemistry resulting from adding water to an unsaturated bond using a mercuric salt catalyst, creating a mercurinium ion intermediate. After formation, this intermediate is attacked by nucleophiles (typically water) to form an unsaturated bond.
Stereochemistry of oxymercuration reactions is defined by several key characteristics.
Here are some highlights:
Markovnikov Addition: Oxymercuration reactions typically exhibit Markovnikov regioselectivity, whereby water serves as the nucleophile that adds hydrogen atoms to carbons of double bonds more heavily substituted with hydrogens. This results in Markovnikov products wherein nucleophile adds only to more heavily substituted carbon atoms of double bonds.
Anti-Addition: Water addition to mercurinium ion intermediates in oxymercuration reactions generally takes the form of anti-addition, in which nucleophiles attach themselves to one carbon of the double bond while the mercuric ion adds itself to another, producing syn addition of the hydroxy group and formation of Hg-O bonds.
Retention of Stereochemistry: Oxymercuration reactions typically result in the preservation of stereochemistry from their starting double bond, meaning if that bond can be classified either geometrically (cis/trans) or stereochemically (E/Z), its configuration will remain intact in the end product.
Stereoselectivity: Oxymercuration reactions can have different stereochemical outcomes depending on various factors, including the nature and composition of their double bonds, substituents attached to them and reaction conditions. Specific substituents or double bond geometries may have an impactful regio- and stereochemical outcome of their reactions.
Oxymercuration reactions follow a stereochemical pathway involving Markovnikov products with anti-addition of nucleophiles and retention of starting double bond stereochemistry. Understanding these stereochemical aspects is essential in order to predict and control oxymercuration reactions with specific regio- and stereochemical transformations in organic synthesis.
Regioselectivity of oxymercuration
Oxymercuration reactions exhibit distinct regioselectivity when adding nucleophiles (usually water) to carbon atoms of unsaturated bonds, unlike acid-catalyzed hydration or hydroboration reactions, for which this reaction might preferentially add nucleophiles such as acid to certain carbon atoms of an unsaturated bond.
Oxymercuration’s regional selectivity can be summarized as follows:
Markovnikov Regioselectivity: Oxymercuration reactions follow Markovnikov regioselectivity, meaning the nucleophile preferentially adds hydrogen atoms to carbons of unsaturated bonds that contain more substituted carbon atoms, leading to formation of products where this nucleophile adds preferentially on more substituted double bonds.
Anti-Markovnikov Hydration: As opposed to acid-catalyzed hydration reactions that involve anti-Markovnikov addition of water, oxymercuration reactions take place with Markovnikov selectivity; using mercuric salt catalysts enables Markovnikov addition of water onto more substituted carbon atoms leading to formation of products containing alcohol groups attached directly onto these carbons atoms.
Regioselectivity in Conjugated Systems: Oxymercuration can display regioselectivity when coupled with conjugated systems, depending on their relative positions of double bonds and substituents, such as electronic effects and steric hindrance between components in the system. Such effects could ultimately influence its outcome.
Regioselectivity observed during oxymercuration reactions is caused by the formation of a cyclic intermediate called the mercurinium ion, created through addition of mercuric salt catalyst to an unsaturated bond followed by attack from nucleophile. Regioselectivity results from its stable yet reactive nature which favors nucleophilic addition onto more substituted carbon atoms.
Understanding the regioselectivity of oxymercuration reactions is essential to synthetic planning and outcomes of reactions, enabling chemists to anticipate and control which products will form from reactions involving this method of activation, providing valuable tools in synthesizing complex molecules or functionalizing specific positions in unsaturated compounds.
What is demercuration?
Demercuration (reductive demercuration) is a chemical process which removes mercury-containing groups or atoms from compounds to convert organomercury compounds to their organic counterparts, typically alcohols.
Demercuration reactions are accomplished by adding a reducing agent such as sodium borohydride (NaBH4) that selectively reduces mercury species to generate the desired organic product.
Demercuration is an effective and crucial process that removes mercury atoms from a compound while maintaining the organic framework. Demercuration plays an integral part in organomercury synthesis and purification processes as well as being utilized frequently for various organic transformations and medicinal chemistry applications.
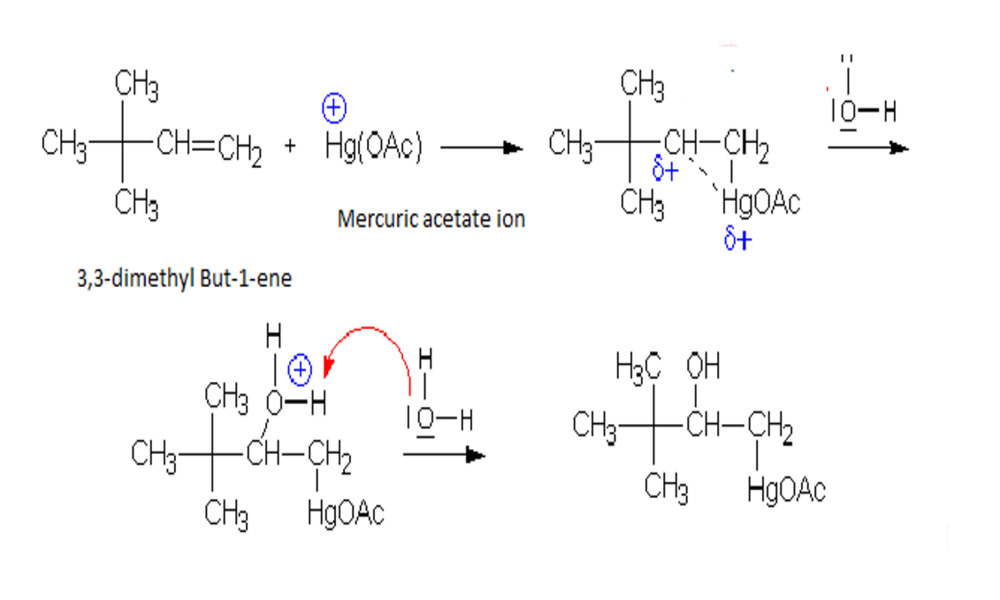
Reduction of mercurinium ion
Reduce mercurinium ion to facilitate demercuration is a critical step in this process. Mercurinium is an intermediate produced during oxymercuration when mercuric salt catalyst adds double bonds of alkene or alkyne compounds; demercuration involves selective removal of mercury-containing groups from this intermediate.
Reducing mercury species typically requires using a mild reducing agent like sodium borohydride (NaBH4). NaBH4 works efficiently while protecting organic structures within molecules during this process; typically the reactions takes place as follows.
Introduction of Reducing Agent: Sodium Borohydride (NaBH4) is added to the reaction mixture containing the mercurinium ion for reduction purposes.
Reducing Mercury Species: NaBH4 donates hydride ions (H-) to mercurinium ion, leading to its reduction. Mercury then undergoes oxidation-reduction reaction from its original state (typically +1) into zero-valent state, leading to metal-hydride intermediate formation.
Formation of Alcohol: Once devoid of its mercury moiety, reduced mercurinium ion undergoes further chemical reactions such as hydrolysis or protonation and finally forms alcohol, thus restoring carbon-carbon double bonds and producing organic products as desired.
Note that any reduction of mercurinium ions must be carried out selectively to avoid unwanted side reactions or over-reduction of functional groups within molecules. A suitable reducing agent and reaction conditions play an essential part in reaching desired results while protecting organic framework integrity.
Demercuration involves the reduction of mercurinium ion to remove mercury-containing groups or atoms and produce organic products, usually an alcohol derivative. This process transforms organomercury compounds into their respective organic derivatives while simultaneously eliminating toxic and potentially hazardous mercury components.
Stereochemistry of demercuration
Demercuration tends to preserve the stereochemical features of organomercury compounds starting points, making this approach particularly helpful when they possess stereochemistry that needs to be preserved in their organic product form.
Demercuration utilizes a reducing agent such as sodium borohydride (NaBH4) to selectively extract mercury-containing molecules from organomercury compounds without significantly altering their stereochemistry or altering any remaining organic framework.
Here are a few key points about demercuration’s stereochemistry.
Retention of Stereochemical Configuration: Reducing mercury-containing species usually does not lead to inversion or alteration of stereochemical configuration of organomercury compounds; rather, selective removal of mercury moiety leaves organic substituents with their original configuration intact.
Stereoisomer Selectivity: Demercuration reactions tend to be stereospecific, or preferring specific stereoisomers of an organomercury compound as they proceed. This selectivity can be affected by factors like mercury atom reactivity and accessibility as well as local steric and electronic effects at the reaction site.
Substrate-Dependent Effects: It is important to recognize that the stereochemical results of demercuration depend on both its substrate and reaction conditions. Certain organomercury compounds may exhibit more sensitive reactions during reduction processes while others can maintain stereochemistry better.
Synthetic Applications: Demercuration’s stereochemical preservation allows chemists to perform selective transformations on organomercury compounds with known stereochemistry and create complex organic molecules with precisely defined stereochemistry synthesis of natural products, pharmaceuticals or any compound in which stereochemical control is essential. It is an invaluable resource in natural product synthesis as well as pharmaceutical compound production.
Demercuration reactions usually retain the stereochemistry of organomercury compounds used as starting materials, making demercuration reactions ideal for manipulating and using chiral organomercury compounds while maintaining stereochemistry integrity within desired organic products.
Regioselectivity of demercuration
Demercuration reactions typically do not display notable regioselectivity, unlike their counterpart oxymercuration which displays this property through water addition to unsaturated bonds. Demercuration focuses on extracting mercury atoms or mercury-containing groups from organic compounds without regard to specific regioselectivity requirements.
Demercuration involves employing a reducing agent such as sodium borohydride (NaBH4) to selectively reduce mercury species present in organomercury compounds. This usually happens without favoring one carbon atom or site over the other in terms of reduction.
However, it should be remembered that demercuration reactions can be affected by both electronic and steric effects around mercury-containing groups. Sometimes accessibility of mercury atoms or groups may alter their reactivity and removal processes – in such instances certain structural features of organomercury compounds may inhibit or improve demercuration processes.
Demercuration does not possess inherent regioselectivity; however, when used in combination with other regioselective reactions or functional group manipulations it can provide desired regioisomeric outcomes. By employing appropriate synthetic strategies chemists can control the regiochemistry of final organic product through transformations following demercuration.
Demercuration processes tend not to be selective with respect to which carbon atom or group the mercury atom or group is removed from. However, using complementary reactions and strategies chemists can achieve selective transformations by combining demercuration with subsequent synthetic steps.
Demercuration of cyclic mercurials
Demercuration of cyclic mercurials refers to the practice of extracting mercury-containing moiety from an otherwise mercury-containing compound through organic synthesis, typically to transform organomercury compounds into their respective organic derivatives.
Demercuration of cyclic mercurials generally follows similar principles to demercuration of acyclic compounds. A reducing agent such as sodium borohydride (NaBH4) is typically employed to selectively reduce mercury species while maintaining their cyclic framework of the molecule.
Demercuration of cyclic mercurials may depend on factors like their ring size, substituents on the ring and accessibility to mercury atoms or groups within its structure; all of these may impact demercuration efficiency and effectiveness.
Noting the difficulty involved with extracting mercury atoms or groups from cyclic mercurials requires taking certain factors and considerations into account. Potential challenges could include potential steric hindrance within the ring, effects on its stability and byproduct formation or side reactions.
Demercuration of cyclic mercurials provides opportunities for the synthesis of various organic compounds, such as alcohols and ketones that contain functionalized cyclic moiety. By selectively removing mercury, chemists gain access to an array of functionalized cyclic molecules which could find use in pharmaceuticals, natural product synthesis, materials science or even materials engineering applications.
Demercuration of cyclic mercurials refers to the selective removal of mercury-containing groups from an organomercury compound through various means, including removal by chemical means such as mercury atom or group removal from its molecular orbitals or organic bonds.
Demercuration process considerations include ring size, substituents and accessibility which affect its efficiency and selection as a demercuration method allowing chemists to design efficient yet selective transformations for synthesizing various organic cyclic organic compounds.
Difference Between Oxymercuration and Demercuration
Below is a comparison table highlighting the key differences between oxymercuration and demercuration:
| Aspect | Oxymercuration | Demercuration |
|---|---|---|
| Definition | Addition of water to unsaturated bonds using a mercuric salt catalyst | Removal of a mercury atom or mercury-containing group from an organic compound |
| Reactants | Alkene or alkyne, mercuric salt catalyst (e.g., Hg(OAc)2) | Organomercury compound, reducing agent (e.g., NaBH4) |
| Reaction Mechanism | Formation of a mercurinium ion, followed by nucleophilic attack by water | Reduction of mercury species to yield organic product |
| Stereochemistry | Generally results in the formation of Markovnikov alcohols (nucleophile adds to the carbon with more hydrogen atoms) | Maintains the stereochemistry of the starting organomercury compound |
| Regioselectivity | Adds water preferentially to the more substituted carbon of the double bond | No specific regioselectivity; mercury moiety is removed from the molecule |
| Selectivity for Functional Groups | Primarily applicable to alkenes and alkynes | Primarily applicable to organomercury compounds |
| Applications | Conversion of alkenes/alkynes to alcohols | Purification and removal of toxic mercury moiety |
| Limitations | Limited scope in terms of applicable substrates | Requires the presence of an organomercury compound |
This comparison table provides an overview of the main differences between oxymercuration and demercuration, including their definitions, reactants, reaction mechanisms, stereochemistry, regioselectivity, selectivity for functional groups, applications, and limitations.
Further exploration of each aspect will help in gaining a comprehensive understanding of the distinctions between these two processes.
Importance of understanding the differences between the Oxymercuration and Demercuration
Understanding the differences between oxymercuration and demercuration are of vital importance in organic chemistry for various reasons:
Selective Functional Group Transformations: Oxymercuration and demercuration offer chemists the capability of selectively adding or removing functional groups in an controlled fashion. By understanding their differences, chemists can select an optimal method depending on their desired transformation, creating efficient yet precise synthetic strategies.
Regoselectivity Control: Oxymercuration shows regioselectivity by adding water preferentially to carbon atoms with more substituted carbons in an alkene or alkyne, providing for specific regioisomers formation and providing predictable reactions results. Understanding this property allows chemists to plan and execute reactions with predictable outcomes.
Stereoselective Reactions: Oxymercuration typically preserves the stereochemistry of an alkene or alkyne starting material, which can be crucial when synthesizing chiral compounds.
By contrast, demercuration does not significantly change its starting organomercury compound’s stereochemistry – so understanding their consequences helps chemists design reactions that maintain or modify desired stereochemical features as desired.
Mercury Removal and Safety: Demercuration is essential for safely eliminating toxic mercury moieties from organic compounds. Organomercury compounds can be highly poisonous and cause environmental issues; with knowledge of demercuration chemists can safely eliminate mercury from compounds to ensure both reaction process and final products safety.
Synthetic Methodology Development: Chemists who understand the unique features and limitations of both oxymercuration and demercuration processes can use this knowledge to create innovative synthetic methodologies. By exploring their differences, chemists may discover opportunities for innovation leading to more efficient and diverse synthetic routes.
Knowing the differences between oxymercuration and demercuration empowers chemists to make informed decisions regarding functional group transformations, regioselectivity control, stereochemistry, safety, and developing new synthetic methodologies. This knowledge increases precision, efficiency, safety, and expands possibilities for creating complex and valuable molecules in organic synthesis.
Future research and advancements in the field
Converting alkenes to alcohols is an ever-evolving field, with ongoing research focused on developing new methodologies, increasing reaction efficiency, and expanding substrate applications. Below are some potential areas of future research and advancement within this field:
Research in Catalyst Development: Scholars are conducting extensive studies on the design and development of novel alkene hydroxylation catalysts, such as new transition metal catalysts or ligands that may enhance reaction rates, selectivity and functional group compatibility.
Selectivity Control: Attaining selective alkene functionalization remains a difficult challenge. Future research seeks to develop strategies to modulate selectivity, enabling the production of specific alcohol isomers from various alkene substrates via catalyst modifications, reaction conditions and group strategies.
Green and Sustainable Approaches: Greener and more sustainable methods for alkene hydration have become an increasing research area, including exploring sustainable solvents, feedstocks and catalysts that minimize or avoid using toxic or environmentally damaging reagents.
Late-Stage Functionalization: Initiatives are underway to broaden the scope of alkene hydration reactions so as to allow late stage functionalization. This would enable direct conversion of readily available alkenes into more advanced synthetic intermediates, simplifying synthetic routes and decreasing steps needed.
Mechanistic Studies: Explorations into the mechanistic aspects of alkene hydration reactions will deepen our knowledge of the reaction pathways, intermediates and selectivity-determining factors – this in turn can guide development of new reaction conditions, catalysts and strategies that allow alkene-to-alcohol conversions with optimal results.
Application in Natural Product Synthesis: Alkene hydration reactions play an integral part in the production of complex natural products, pharmaceutical intermediates and bioactive compounds. Future research should leverage alkene to alcohol conversions to optimize natural product synthesis efficiently and cost-effectively.
Computational Approaches: Computational methods such as quantum chemical calculations and molecular modeling are increasingly being employed to gain insights into reaction mechanisms, predict selectivity, and guide catalyst design. With further advancement of computational tools comes an accelerated discovery and optimization of alkene hydration reactions.
Future research directions and advances may significantly enhance the efficiency, selectivity, and sustainability of alkene to alcohol conversions. By expanding existing methodologies and understanding their underlying principles, researchers may be able to unlock more synthetic potential of this transformation process for various applications like organic synthesis, pharmaceuticals research and materials science.
Conclusion
Converting alkenes to alcohols is an essential process in organic chemistry that introduces an hydroxyl group onto carbon-carbon double bonds. Two common methods for accomplishing this conversion are acid-catalyzed hydration and oxymercuration-demercuration.
Understanding the differences between acid-catalyzed hydration and oxymercuration-demercuration processes is vital to selecting an appropriate method based on factors like regioselectivity, stereochemistry and specific requirements for desired product. Acid-catalyzed hydration follows Markovnikov regioselectivity by adding water across double bonds; while oxymercuration-demercuration proceeds via formation of mercurinium ion intermediate and allows anti-Markovnikov addition of water across double bonds.