Introduction of Kelvin and Fahrenheit
An introduction is the initial section of any piece of writing which introduces its topic and provides some background. A successful introduction should grab reader’s attention and make them want to continue reading further.
When writing an Introduction, there are a few key Considerations to keep in mind:
Start with an eye-catching sentence or paragraph, such as a quote, statistic or question that captures attention. Next, provide some background information – this could include definitions, histories or summarizing the main points.
State your thesis statement clearly. A thesis statement serves as the main idea behind your paper; it should be clear, concise, and arguable. Outline the main points of your paper so as to give readers an overview of what to expect in their reading experience. Toward this end, here’s an example of an introduction:
Ray Bradbury’s Fahrenheit 451 paints a dystopian future where books have been banned and firemen are responsible for burning them.
Guy Montag, one of these firemen, comes to question government motives after meeting Clarisse McClellan; Clarisse challenges his beliefs and encourages him to think for himself; as Montag reads books himself he discovers that government lies about everything; thus prompting him to rebel against authority by helping other escape the firemen’s reach.
This introduction is effective because:
Starts off with an attention-getting sentence. provides some background on the topic at hand. states thesis statement.and finally provides an outline of main points covered within paper. By following these tips, you can compose an introduction that will grab the reader’s attention and encourage them to continue reading.
What are Kelvin?
Kelvin is the unit of thermodynamic temperature used within the International System of Units (SI). It was named for William Thomson, 1st Baron Kelvin (1824-1907). The Kelvin is defined such that zero K is absolute zero, the temperature at which all thermal motion stops.
A change of thermodynamic temperature by one Kelvin corresponds to an increase or decrease of thermal energy by 1.380649×10-23 J; Boltzmann constant k = 1.380649×10-23 JK-1 was defined exactly in 2019 redefinition of SI base units such that triple point of water lies at 273.16+-0.0001 K.
Kelvins are used in combination with their prefixed forms such as millikelvin (mK), microkelvin (uK) and nanokelvin (nK) to measure very small changes in temperature such as those encountered in physical experiments and Earth’s atmosphere. These units serve to monitor very minute shifts that occur over time.
Kelvins can also be used to measure the temperatures of stars and other celestial bodies; Sun’s surface temperature, for instance, stands at approximately 5,800 K. Kelvins are an invaluable measurement unit used throughout science and engineering, measuring temperatures from the tiniest atoms up to vast galaxies in stars.

What are Fahrenheit?
Fahrenheit is a temperature scale developed in 1724 by Daniel Gabriel Fahrenheit (1686-1736). It uses the degree Fahrenheit (symbol: degF) as its unit.
There are various accounts as to how Daniel Gabriel Fahrenheit initially established this scale; his original paper suggests it as being determined as the freezing point for brine solutions composed of water, ice and ammonium chloride salt (an ammonium chloride salt solution).
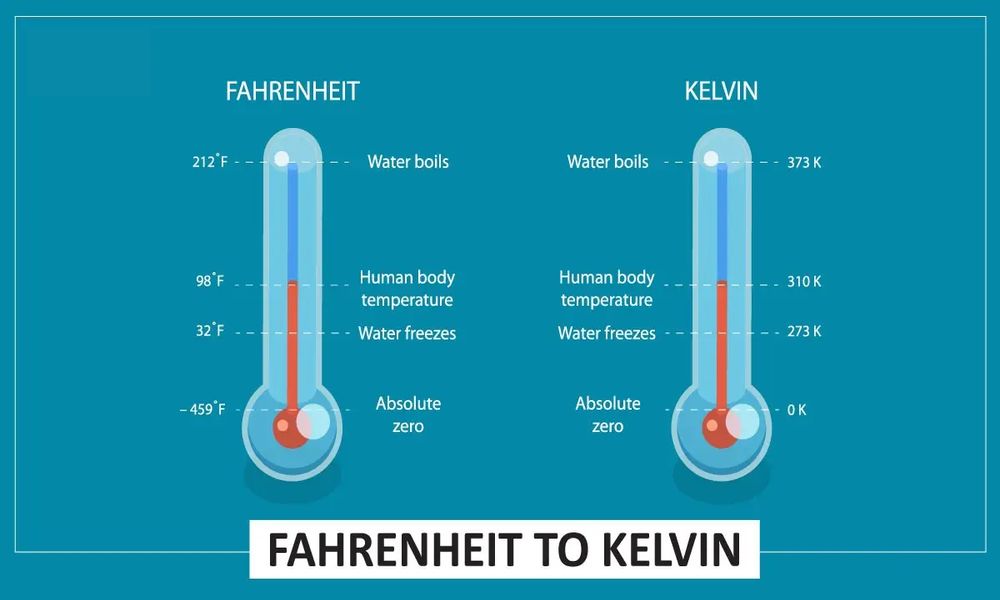
On the Fahrenheit scale, water’s freezing point is 32degF while its boiling point stands at 212degF – this difference being divided by 180 degrees so each degree Fahrenheit corresponds with 1.8 degrees Celsius.
Fahrenheit is the standard temperature scale in the United States. Additionally, it’s used by other nations such as Bahamas, Belize, Cayman Islands Liberia and Philippines.
Fahrenheit offers several distinct advantages over Celsius for measuring temperature. First, its precision spans an expansive temperature range. Second, most people in the United States understand its use as it’s familiar. Thirdly, conversion between Fahrenheit and Celsius is straightforward.
Fahrenheit also has some drawbacks that should be taken into consideration, however. First and foremost, it does not start from absolute zero as its starting point. Furthermore, since Fahrenheit is not widely used around the world it may be challenging to convert between Fahrenheit and other temperature scales.
Overall, Fahrenheit provides an effective temperature scale for everyday use in the United States. It is accurate, familiar and straightforward to convert between other temperature scales – yet is still widely used worldwide. Unfortunately though it cannot be considered an absolute scale.

Comparison Table of Kelvin and Fahrenheit
Sure! Here’s a comparison table highlighting the key differences between the Kelvin and Fahrenheit temperature scales:
| Aspect | Kelvin Scale | Fahrenheit Scale |
|---|---|---|
| Zero Point | Absolute zero (-273.15°C) | Lowest recorded temperature (-459.67°F) |
| Freezing Point | 273.15 Kelvin (0°C) | 32 degrees Fahrenheit (0°F) |
| Boiling Point | 373.15 Kelvin (100°C) | 212 degrees Fahrenheit (100°F) |
| Increment Size | 1 Kelvin | 1 degree Fahrenheit |
| Conversion Formula | Celsius = Kelvin – 273.15 | Celsius = (Fahrenheit – 32) / 1.8 |
| Origin | Based on absolute zero | Based on freezing and boiling points |
| Common Usage | Scientific and engineering fields | United States and a few other countries |
| Advantages | Reflects absolute temperature | Familiar to people in certain regions |
| Disadvantages | Not as widely used in daily life | Less universally understood |
Please note that the conversion formulas mentioned are simplified versions and may not account for precise rounding or significant figures.
History of Kelvin
The Kelvin scale is a temperature scale based on the theoretical concept of absolute zero, or the temperature at which all molecular movement ceases. It was created in the mid 19th century by Scottish physicist and engineer William Thomson, 1st Baron Kelvin.
Thomson’s work was founded on the theory that temperature is an indicator of average molecular kinetic energy, meaning as temperature decreases so does average molecular kinetic energy; at absolute zero this level drops below zero as more molecules become motionless.
The Kelvin scale can be defined as the scale in which absolute zero becomes zero. It is divided into degrees Kelvin (K), with each degree equalling one degree Celsius. Water has a freezing point temperature of 273.15 K while its boiling point temperature stands at 373.15K.
Kelvin scale is the standard temperature scale in scientific work and engineering applications. While Celsius scale is more widely used everyday life, both are ultimately derived from Kelvin.
The Kelvin scale is an invaluable asset to scientists and engineers, allowing them to accurately measure temperatures using it as a reference standard. Furthermore, its use provides insight into understanding matter at extreme temperatures.
Here are a few key events in the history of Kelvin scale:
1709: Daniel Fahrenheit created the first mercury thermometer. 1742: Anders Celsius established his temperature scale (the Celsius scale). 1848: William Thomson, 1st Baron Kelvin published his paper outlining an absolute temperature scale.
1868: The International Meteorological Organization adopts the Kelvin scale as its official temperature scale for scientific work. 1954: The International Bureau of Weights and Measures defines it in terms of triple point of water.
2019: The International System of Units (SI) redefines the Kelvin scale in terms of Boltzmann constant.
History of Fahrenheit
The Kelvin scale is a temperature scale based on the theoretical concept of absolute zero, or the temperature at which molecular motion ceases. Developed in Scotland during the mid 19th century by William Thomson, 1st Baron Kelvin.
Thomson’s work was founded on the notion that temperature is a measurement of average molecular kinetic energy. As temperature decreases, so too does its average molecular kinetic energy decrease – reaching zero at absolute zero.
The Kelvin scale can be understood as the scale on which absolute zero temperature lies at zero. It is measured in degrees Kelvin (K), each K representing one degree Celsius. Water freezes at 273.15 K and boils at 373.15 K respectively.
Kelvin scale is the primary temperature scale used in scientific work and certain engineering applications, while Celsius scale is generally more widely utilized; both scales ultimately derived from Kelvin.
The Kelvin scale is an invaluable asset to scientists and engineers. It allows them to precisely and reliably measure temperatures as well as understand their behavior at extreme temperatures. The Kelvin scale provides essential measurements that enable precise temperature comparison. Its importance cannot be overstated.
Here are a few key moments from the history of Kelvin Scale:
1709: Daniel Fahrenheit creates the first mercury thermometer. 1742: Anders Celsius introduces his Celsius Temperature Scale. In 1848 William Thomson 1st Baron Kelvin publishes a paper outlining an Absolute Temperature Scale.
1868: The International Meteorological Organization adopts the Kelvin scale as its official temperature scale for scientific work. 1954: The International Bureau of Weights and Measures defines it in terms of triple point of water.
2019: The International System of Units (SI) redefines Kelvin scale in terms of Boltzmann constant. See profile picture and history of Fahrenheit here for further drafts of Fahrenheit scale definitions.
1 The Fahrenheit scale was first proposed in 1724 by Daniel Gabriel Fahrenheit (1686-1736).
It utilizes degrees Fahrenheit (symbol: degF). There are various accounts as to how he first defined this scale; his original paper suggests the lower defining point, 0 degF, was chosen as the freezing temperature of brine made by mixing water, ice and ammonium chloride salt together, with his upper defining point (100 degF) serving as his own body temperature; over time he adjusted it such that 32 degF was set as its freezing point with 180 degF between freezing point and boiling point with just 180 deg between these points (the upper limit being at 100 degF).
Fahrenheit was born in Gdansk, Poland in 1686. After moving to Amsterdam for his studies of physics and mathematics in 1708, he created his revolutionary mercury thermometer which proved more accurate than alcohol thermometers then in use – ultimately creating the Fahrenheit scale using his invention.
Fahrenheit was commonly used until the middle of the 20th century, when its usage slowly gave way to Celsius as more easily reproducible references points than Fahrenheit could provide.
Today, Fahrenheit scale is still used in certain parts of the US such as weather forecasting or cooking recipes; however, Celsius scale is far more prevalent globally.
Here are some key milestones in the history of Fahrenheit scale:
1709: Daniel Fahrenheit invented the first mercury thermometer. 1724, Fahrenheit proposed his Fahrenheit temperature scale; 1742 saw Anders Celsius propose his Celsius scale instead.
1868: The International Meteorological Organization adopts Celsius scale as the official temperature scale for scientific work. 1954: International Bureau of Weights and Measures defines Celsius scale in terms of its triple point water equivalent.
2019: The International System of Units (SI) redefines Celsius on terms of Boltzmann constant.
Scientific and engineering applications using Kelvin scale
The Kelvin scale is a temperature scale based on the theoretical concept of absolute zero, or the temperature at which all molecular motion ceases. Developed by William Thomson, 1st Baron Kelvin from Scotland during the mid 19th century.
The Kelvin scale can be defined as the temperature scale on which absolute zero temperature equals zero, divided into degrees Kelvin (K), with each degree representing one degree Celsius. Water’s freezing point is set at 273.15K while boiling point stands at 373.15K.
Kelvin scale is the preferred temperature scale used in scientific work and some engineering applications. While Celsius scale is more widely utilized for everyday usage, both are ultimately derived from Kelvin.
The Kelvin scale is a vital tool for scientists and engineers, enabling them to accurately measure temperatures with consistency across measurements. Furthermore, its measurements give scientists insight into how matter behaves at extreme temperatures.
Here are a few scientific and engineering applications of the Kelvin scale:
Thermodynamics: Thermodynamics is the study of how heat interacts with other forms of energy. To better understand thermodynamics, the Kelvin scale provides scientists with a means of measuring matter temperatures at extreme temperatures.
Astrophysics: Astrophysics is the study of physical properties found within stars, galaxies, and other celestial objects in space. Kelvin scale is essential in this field as it allows scientists to accurately measure star temperatures.
Engineering: Engineering involves applying scientific knowledge to practical problems. The Kelvin scale is essential in engineering as it allows designers and builders to design structures and machines capable of withstanding extreme temperatures.
The Kelvin scale is an invaluable instrument used across numerous scientific and engineering applications. As an accurate way of measuring temperatures, the Kelvin scale plays a critical role in understanding matter at extreme temperatures.
Everyday use cases and familiarity with Fahrenheit scale
The Fahrenheit scale, created in 1724 by German physicist Daniel Gabriel Fahrenheit and commonly used throughout North America and other parts of Europe, measures temperature. With 180 degrees between 32 and 212, its use provides a convenient temperature scale.
The Fahrenheit scale is widely utilized across American society for many different applications, such as:
Weather forecasting: The National Weather Service uses Fahrenheit scale to report temperature, humidity and other atmospheric conditions. Clothing retailers in the US employ it for labeling temperature ranges for various clothing items.
Cooking: United States recipes often use Fahrenheit scale as their standard way to write them out, and medical care providers likewise employ it when taking body temperature readings.
Fahrenheit scale is also used in some other countries, including Bahamas, Belize, Cayman Islands, Liberia and United Kingdom; however most countries worldwide prefer Celsius scale which measures freezing and boiling points at sea level.
The Celsius scale has become more widespread since its scientific definition is more universally accepted than that of Fahrenheit.
Fahrenheit relies on freezing and boiling points of water at specific atmospheric pressure, which may differ significantly depending on where one lives in the world. Meanwhile, Celsius tracks these points using freezing and boiling points at sea level as its reference standard – making for easier comparisons across countries and cultures.
Although scientifically advantageous, Fahrenheit remains popular in the US as its use has become habitual. A shift to Celsius would cause substantial disruption, so no real incentive exists to switch over.
Unique characteristics and usage of the Fahrenheit scale
The Fahrenheit scale, created in 1724 by German physicist Daniel Gabriel Fahrenheit, is a temperature scale widely used throughout the United States and some other countries. This temperature scale features 180 degrees between its freezing point (32 degrees) and boiling point of water, or its freezing and boiling points respectively.
The Fahrenheit scale stands out in several ways. First, it is the only standard temperature scale based on brine temperature fluctuations – an emulsion of water and salt – between freezing and boiling points.
Furthermore, the Fahrenheit scale provides more accurate readings for temperatures both very cold and extremely hot than its Celsius equivalent, giving users access to temperature measurements at both ends of its range.
The Fahrenheit scale is used in numerous everyday applications in the US. For instance, National Weather Service utilizes it to report temperatures, humidity levels, and other weather conditions; clothing retailers label temperature ranges of clothing items with Fahrenheit scale; recipes written using Fahrenheit are often written using Fahrenheit; while doctors and nurses employ it when measuring body temperatures.
Although Fahrenheit scale has its own distinctive qualities, it is slowly being phased out in favor of Celsius scale in the United States due to the latter’s more scientific definition of temperature and wider use throughout the globe. Still, Fahrenheit scale will likely remain popular as people are familiar with it.
Here are some of the unique properties and uses of Fahrenheit scale:
The Fahrenheit scale measures water at 32 degrees, with boiling point sitting at 212. The gap between freezing and boiling points of water on this scale is 180 degrees.
The Fahrenheit scale is based on the freezing and boiling points of brine, a mixture of water and salt. The Fahrenheit scale has a wider temperature range than Celsius scale and is widely used throughout everyday applications in the US.
In the United States, Celsius scale is gradually being adopted to replace Fahrenheit scale.
Zero points and absolute temperature
The zero point on any temperature scale represents the point at which temperatures reach zero on that scale, while absolute zero represents the lowest possible temperature that can be achieved on all temperature scales; all movement stops and all temperature scales read off zero simultaneously.
The zero points for different temperature scales differ. For instance, on the Fahrenheit scale it’s 32 degrees Fahrenheit while for Celsius it is zero degrees Celsius – however the absolute zero remains the same on each.
Absolute temperature is a measure of the kinetic energy of particles in a system; as absolute temperature rises, more rapidly the particles move. Absolute zero marks the point at which all kinetic energy has vanished and all movement ceases.
Absolute temperature is measured in Kelvins (K). The freezing point of water is 273.15 K while its boiling point stands at 373.15 K – absolute zero is defined as zero Kelvins.
Kelvin scale is the primary means by which absolute temperature can be measured, and is based on the triple point of water, or 273.16 K as defined by Wikipedia. This triple point can exist as solid, liquid and gas form in equilibrium at that specific temperature and pressure combination.
The Rankine Scale is used to measure absolute temperature. Based on Fahrenheit scale, with freezing point being 492.22 R and boiling point being 671.67 R respectively; absolute zero is defined as zero R.
Kelvin and Rankine scales are absolute scales, which means that their zero points lie at absolute zero. This makes them useful scientific measurements as they can be used to gauge temperatures that range from extremely low to extremely high levels.
Advantages and disadvantages of each scale
There are various kinds of scales, each offering distinct advantages and disadvantages. Some of the more commonly used ones include:
Spring scales: Spring scales work by measuring the force required to stretch a spring. While they’re fairly inexpensive and straightforward to use, their accuracy could vary if your spring is not calibrated correctly.
Balance scales: Balance scales work by comparing an object’s weight against that of a known reference object. While more accurate than spring scales, balance scales may require additional space and be more challenging to use.
Digital scales: Digital scales utilize electronic sensors to measure weight accurately and are therefore considered the most accurate type of scale; however, these scales tend to be more costly than their alternatives.
The best type of scale for any given application depends on the needs and desires of its user. A spring scale might suffice when measuring weight of small objects while balance scales may be necessary when weighing heavy ones.
Here is an in-depth examination of each type of scale:
Spring scales offer several advantages: Inexpensive, portable and easy to use; plus they require no batteries for operation and accurate measurements are easy to read on portable platforms; they’re lightweight too – all essential features in today’s business world.
But spring scales also come with some drawbacks: Can be inaccurate when not calibrated properly (unlike other scales); not as accurate compared with balance and digital scales and may need batteries; less space-efficient than spring ones requiring batteries (balance scales are less accurate than other forms of scales); plus the latter require batteries (though more difficult in use); more difficult operation requires more space; needs more space-consuming batteries (balance scales do not), less space required per scale used (unlike spring ones); more difficult use, more difficult use requires batteries; requires batteries (unless not powered digital).
Digital scales offer the most accurate type of scale available with easy readability (although expensive than spring ones); easily portable costs (although also portable); disadvantages (compared with spring ones) due to cost effectiveness (due to zeroing)/can cost prohibitively priced as iterators/dea/can cost prohibitively priced to use and may require calibration/calibation in comparison).
Requires batteries Beyond choosing the appropriate scale model, other factors can impact its accuracy as well, including environment factors. Scales should be placed on level surfaces without sources of vibration and should also be calibrated prior to taking measurements.
Practical Examples and Applications
Here are a few practical applications of scales:
Spring scales are commonly seen in retail stores to measure produce and other items, and in laboratories to accurately weigh chemicals or other materials.
Balance scales are widely used in jewelry stores to weigh precious metals and gemstones. Additionally, these scales can also be employed in scientific research for measuring the weight of small particles.
Digital scales are widely used at home to measure food and other items, as well as in gyms and fitness centers for monitoring bodyweight.
Scales have multiple practical uses beyond this field of practicalities; for instance, engineers use scales for designing and building bridges while doctors utilize them to monitor patient weight and manufacturers ensure their products comply with weight specifications.
Recap of key points discussed
Here is an outline of key points discussed:
There are various kinds of scales, each offering specific advantages and disadvantages for use in specific applications. To select the ideal scale for any given job, the user will have to take their specific needs into consideration.
Additionally to scale type, environmental conditions also play a part in the accuracy of measurements taken. Scales are used in various practical settings, including retail stores, laboratories, jewelry stores, homes, gyms and fitness centers. Scales also play a prominent role in engineering, medicine and manufacturing applications.
Conclusion
Kelvin and Fahrenheit temperature scales each have unique properties and applications. Kelvin scale is based on absolute zero and commonly used by scientists and engineers for precise temperature measurement systems. Fahrenheit scale is predominantly used within the United States as it uses freezing and boiling points of water as its basis, thus being more familiar to residents in those regions.
The Kelvin scale begins at absolute zero, which is defined as the temperature at which all molecular movement ceases. By contrast, Fahrenheit uses its lowest recorded temperature as its zero point; freezing and boiling points also differ between scales: on Kelvin it corresponds to 32-212F on Fahrenheit respectively.