Introduction
Refining crude oil plays an essential part in meeting society’s evolving demands for various useful products. Of the various refining processes employed, catalytic cracking and hydrocracking stand out as key techniques used to convert heavy hydrocarbon feedstocks to more valuable gasoline, diesel and petrochemical products.
Catalytic Cracking and Hydrocracking are highly developed processes that use catalysts to facilitate specific chemical reactions. Although both processes involve breaking apart heavy hydrocarbon molecules, their specific objectives, reaction mechanisms, feedstock requirements, product yields and environmental considerations vary widely.
This content outline explores the inner workings of catalytic cracking and hydrocracking processes, exploring their definitions, principles, process descriptions, catalysts used during reactions and their resulting product profiles as well as any advantages or disadvantages they might present for each. Furthermore, we will discuss any industrial applications they might present for consideration.
Catalytic cracking and hydrocracking will be thoroughly compared, highlighting their distinct features that set them apart. We will consider factors like feedstock flexibility, product quality variances, catalyst characteristics, energy requirements, environmental impacts and areas of application for both processes.
Recognizing the differences between catalytic cracking and hydrocracking is essential for refinery operators, engineers, and industry professionals, as it allows them to optimize operations, select an ideal process for specific feedstocks, and maximize production of valuable fuels and petrochemicals.
Catalytic Cracking and Hydrocrackingprovide important refining processes, and by studying their details we can gain greater insights into them as well as anticipate any advancements or innovations that might take place in this sector.
Catalytic Cracking
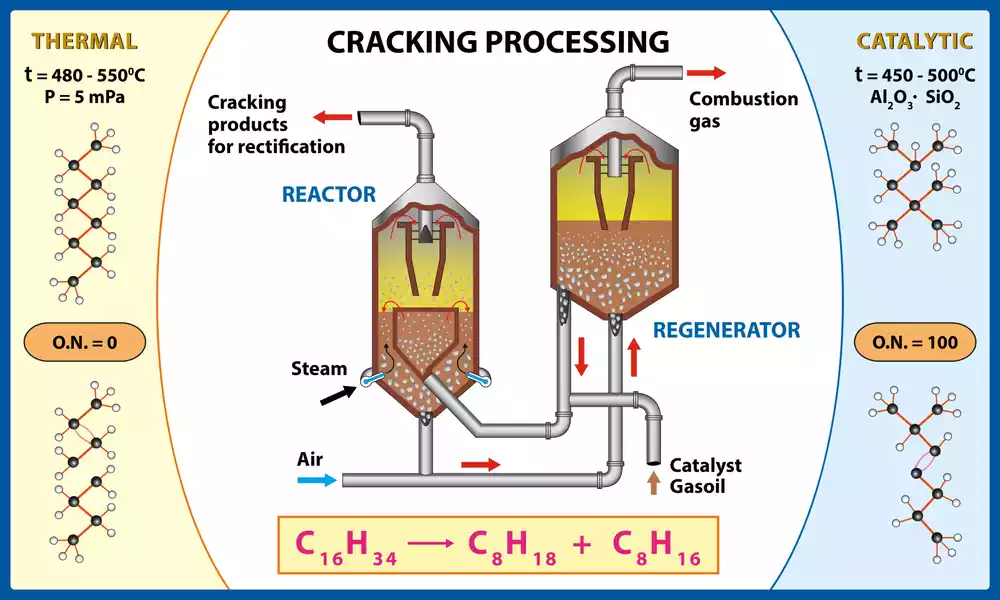
Catalytic cracking is a refining process employed by the petroleum industry to convert heavy hydrocarbon molecules into lighter and more valuable products. A type of thermal cracking, it utilizes a catalyst to accelerate chemical reactions during this process while simultaneously lowering temperatures needed for these reactions, thus increasing efficiency and selectivity in cracking operations.
Catalytic cracking involves mixing high-boiling hydrocarbon feedstocks such as heavy gas oil or vacuum residue with powdered catalysts made up of zeolites or acidic materials to form an inorganic catalyst mixture, before heating and vaporizing before entering a catalytic cracking unit known as either a fluidized bed reactor or riser reactor for cracking.
Inside the reactor, hot vaporized feedstock comes into contact with a catalyst and sparks off a series of cracking reactions. The catalyst provides a surface on which hydrocarbon molecules can react and break their carbon-carbon bonds to form smaller molecules; often leading to the creation of olefins, gasoline-range hydrocarbons and lighter gases during these reactions.
Cracked products, along with their spent catalyst, are then separated. Regenerating of the catalyst takes place by burning off coke formed during cracking reactions; once done, this regenerated catalyst can then be returned back into the reactor for further cracking reactions.
Catalytic cracking is an integral step of petroleum refining that enables heavy feedstocks to be converted to lighter, higher value products like gasoline, diesel and petrochemical feedstocks – an essential step for producing transportation fuels.
Advantages and disadvantages of catalytic cracking
Advantages of Catalytic Cracking:
Catalytic Cracking Increases Product Yield: Catalytic cracking can transform heavy hydrocarbon feedstocks into lighter and more desirable products such as gasoline, olefins and petrochemical feedstocks compared to traditional refining processes, providing greater yield of desirable products compared to alternative refining processes.
Feedstock Flexibility: Catalytic cracking allows refineries to utilize various types of crude oil in their operations and maximize operational efficiencies with greater ease, thanks to its ability to process diverse feedstocks such as heavy gas oils and vacuum residues. This enables refineries to capitalize on their efficiency.
Production of Gasoline: Catalytic cracking has proven itself especially successful at producing gasoline, an essential transportation fuel. Catalytic cracking helps meet the increasing demands for this commodity in the automotive industry.
Catalyst Regeneration: Catalytic cracking catalyst can be regenerated through burning off any excess coke produced during cracking reactions, providing continuous use and decreasing replacement needs. This regeneration process ensures constant renewal while eliminating frequent replacement costs.
Catalytic Cracking Has Its Drawbacks:
Product Quality: Catalytic cracking typically produces lower-grade products compared to other refining processes, like hydrocracking. More olefins and less hydrogenated compounds may compromise product stability and performance in catalytic cracking products.
Environmental Effects: Catalytic cracking can produce emissions and byproducts that contribute to air pollution, necessitating additional environmental controls and treatment measures for its operation.
Energy Requirements: Though catalytic cracking generally has lower energy requirements compared to processes like hydrocracking, it still requires significant energy inputs primarily for heating and vaporizing feedstocks.
Catalyst Deactivation: Over time, catalytic cracking catalysts may become deactivated due to fouling or poisoning and require regular regeneration or replacement services for maximum performance – this adds up to increased operational and maintenance costs associated with catalytic cracking processes.
Notable here are that the advantages and disadvantages outlined above are general observations; their exact application to your refinery configurations, catalyst formulations, feedstock quality requirements, or operational conditions may vary considerably.
Products obtained from catalytic cracking
Catalytic cracking is a chemical refining process that produces various high-value products, depending on a number of variables such as feedstock used, operating conditions and catalyst formulation. Here are some typical products resulting from catalytic cracking:
Gasoline: Catalytic cracking processes have long been recognized for producing large quantities of gasoline, an essential transportation fuel. Gas obtained through catalytic cracking processes tends to possess desirable qualities like higher octane rating and better combustion characteristics, enhancing their appeal as an economical source.
Olefins: Catalytic cracking produces large quantities of olefins such as ethylene and propylene. Olefins serve as important building blocks in the petrochemical industry, used to manufacture plastics, synthetic fibers, solvents and other chemicals.
Cracking reactions produce lighter hydrocarbon gases such as methane, ethane, propane and butane that can be used either directly as fuel sources or feedstock for other refining or petrochemical processes.
LPG (Liquefied Petroleum Gas): Catalytic cracking can produce LPG, consisting primarily of propane and butane. LPG has many applications for residential and commercial heating systems, cooking stoves and vehicles alike.
Catalytic Cracking yields some diesel-range hydrocarbons; however, their quality and yield may be inferior compared to dedicated production processes like hydrocracking.
Petrochemical Feedstocks: Catalytic cracking can produce valuable petrochemical feedstocks such as benzene, toluene and xylene (BTX), which are essential ingredients in producing plastics, synthetic fibers, rubbers, solvents and other chemical intermediates.
Product yields and composition can be tailored based on specific process conditions, catalyst selection and refining objectives. Refineries often optimize operations to create the most valuable products based on market demand and profitability.
Feedstock types used in catalytic cracking
Catalytic cracking (CC) is an efficient refining method capable of processing different feedstocks. For example:
Heavy Gas Oils: Distilled crude oil produces heavy gas oils which are commonly used as feedstock for catalytic cracking reactions. These heavy hydrocarbon molecules typically possess higher boiling points and make for ideal candidates in cracking reactions.
Vacuum Gas Oils: Vacuum gas oils are produced through vacuum distillation of crude oil. Their distinctive features are high boiling points and molecular weight, making them suitable for catalytic cracking processes.
Residues: Residues are heavy, high-boiling fractions left over from crude oil distillation that contain complex hydrocarbon compounds requiring further processing to convert them to valuable products. Catalytic cracking may be employed to upgrade these residues into lighter hydrocarbon fractions that have greater value.
Heavy Atmospheric Distillation Bottoms (HADBs): Heavy Atmospheric Distillation Bottoms are heavy fractions obtained during atmospheric distillation column refinement to produce refined crude oil, with hydrocarbon molecules that can be processed using catalytic cracking as feedstock.
Decant Oils: Decant oils are typically heavy hydrocarbon streams byproducts from other refining processes, such as solvent extraction or deasphalting, that can serve as feedstocks for catalytic cracking.
Keep in mind that selecting feedstock depends on various factors including refinery configuration, product demands, crude oil availability and desired product slate. Refineries often choose feedstocks that provide maximum yield and quality while simultaneously optimizing their operations.
Hydrocracking
Hydrocracking is a refining process employed by the petroleum industry to transform heavy, high-boiling hydrocarbon feedstocks into lighter and more valuable products. It utilizes hydrogenation (the addition of hydrogen) and catalytic cracking processes. Hydrocracking is typically employed to produce superior transportation fuels such as gasoline and diesel with enhanced performance characteristics.
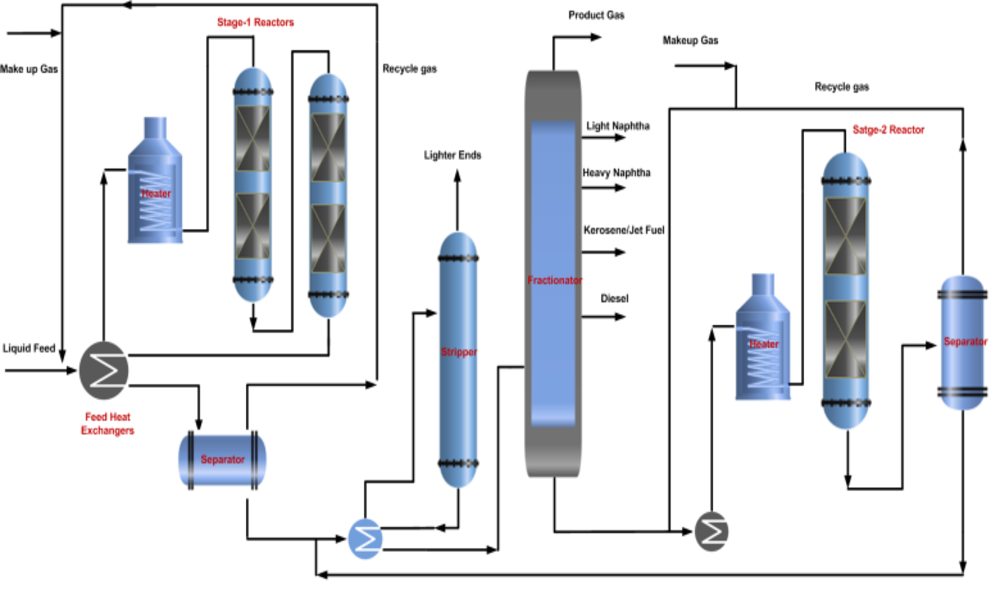
Hydrocracking involves mixing feedstock such as heavy gas oils, vacuum gas oils or residue from other refining processes with hydrogen gas and adding them into a reactor vessel with an appropriate catalyst, such as platinum or palladium supported on high surface area materials like alumina or silica.
Inside the reactor, feedstock and hydrogen undergo a series of chemical reactions catalyzed by the catalyst. Hydrogen molecules interact with hydrocarbon molecules by breaking their carbon-carbon bonds and replacing them with hydrogen atoms; this hydrogenation step helps remove impurities such as sulfur, nitrogen, and metals while simultaneously decreasing molecular weight of hydrocarbons.
Cracking reactions take place simultaneously with hydrogenation. A catalyst facilitates the breaking of carbon-carbon bonds to form lighter hydrocarbon molecules; cracking reactions lead to gasoline, diesel, jet fuel and petrochemical feedstocks being formed as products from this reaction process.
Once reactions have completed, the mixture of cracked products, unreacted hydrocarbons, and excess hydrogen is separated and returned back into the reactor for further processing. Meanwhile, cracked products will undergo additional refining steps such as distillation in order to yield fuels or other valuable products.
Hydrocracking offers numerous advantages, such as increased product quality and yield of valuable fuels; expanded feedstock processing options; removal of impurities that reduce environmental impacts; and enhanced environmental and performance characteristics of final products.
Advantages and disadvantages of hydrocracking
Hydrocracking offers several advantages:
Hydrocracking Delivers High-Quality Product Yield: Hydrocracking creates superior products when compared to catalytic cracking. Hydrocracking’s hydrogenation of hydrocarbon molecules results in lower sulfur, nitrogen and aromatics content which improves both environmental performance and fuel performance characteristics of final fuel products.
Increased Diesel Production: Hydrocracking has proven itself particularly efficient at producing diesel fuel, meeting rising demands in transportation and industrial sectors for it while producing cleaner-burning, lower sulfur diesel fuels.
Hydrocracking provides environmental advantages: by reducing emissions such as sulfur oxides (SOx), nitrogen oxides (NOx) and particulate matter emissions, hydrocracking helps meet stringent environmental regulations while producing cleaner fuels with lower impact on air quality.
Feedstock Flexibility: Hydrocracking can handle a range of heavy feedstocks, such as heavy gas oils, vacuum gas oils and residues, providing refineries with greater adaptability to accommodate different crude oil qualities and maximize their feedstock utilization.
Hydrocracking Has Its Drawbacks:
Hydrocracking Requires Higher Energy Consumption: Due to the high temperatures and pressures involved, hydrocracking requires significant amounts of energy inputs; its energy-intensive nature may impact operational costs in refineries.
Catalyst Deactivation: Hydrocracking metal catalysts are vulnerable to deactivation over time due to fouling, poisoning or sintering; periodic regeneration or replacement may be required in order to keep process efficiency high, thereby increasing operational costs.
Process Complexity: Hydrocracking is more complicated than catalytic cracking due to the higher temperature and pressure conditions as well as hydrogen gas needs, necessitating sophisticated equipment and control systems and making this method both capital-intensive and operationally challenging.
Hydrocracking’s Lower Gasoline Yield: When compared with catalytic cracking, hydrocracking typically results in lower yields of gasoline and lighter hydrocarbon fractions; this could be detrimental if there is higher demand for gasoline versus diesel or additional processing steps are necessary to meet such demands.
As is to be expected, these advantages and disadvantages should only be seen as general observations, with any exact observations depending on your refinery configuration, catalyst formulations, feedstock qualities, operational conditions or specific operational demands.
Products obtained from hydrocracking
Hydrocracking is a refining process that produces various valuable products through hydrogenation and cracking of heavy hydrocarbon feedstocks, producing various valuable products through hydrogenation and cracking processes. The products obtained may vary depending on factors like feedstock type, operating conditions and catalyst formulation – here are some typical hydrocracking outputs:
Hydrocracking is best known for producing diesel fuel at an exceptionally high yield. The process transforms heavy hydrocarbons into lighter, low-sulfur diesel with improved cetane number and enhanced performance characteristics, creating an abundance of cheap and environmentally-friendly diesel.
Hydrocracking produces jet fuel or aviation turbine fuel (ATF), meeting stringent aviation specifications. This process removes impurities while improving overall quality. Hydrocracking can produce LPG (Liquefied Petroleum Gas). LPG is comprised primarily of propane and butane and has many applications, including heating, cooking, and vehicle fuel. Hydrocracking may produce gasoline-range hydrocarbons; however, their yield tends to be less than other methods designed specifically for gasoline production such as catalytic cracking.
Petrochemical Feedstocks: Hydrocracking can produce valuable petrochemical feedstocks such as benzene, toluene and xylene (BTX), which serve as key building blocks in the manufacture of plastics, synthetic fibers, rubbers, solvents and other chemical intermediates.
Lubricant Base Stocks: Hydrocracking can produce high-grade lubricant base stocks with enhanced viscosity, oxidation stability and thermal properties that form the basis for producing various oils and greases. These base stocks serve as the foundation for this production process.
Hydrocracking’s product yields and composition can be tailored by controlling operating conditions, catalyst selection and feedstock properties. Refineries often tailor their hydrocracking operations to meet market demands while maximizing production based on profitability and value considerations.
Feedstock types used in hydrocracking
Hydrocracking is an industrial process used for refining heavy hydrocarbon feedstocks. The specific feedstock types utilized can vary based on refinery configuration, market demands, and desired product slate – here are some commonly utilized by hydrocracking:
Heavy Gas Oils: Heavily refined gas oils derived from atmospheric or vacuum distillation of crude oil are frequently used as feedstock for hydrocracking processes. With high boiling points and large concentrations of heavy hydrocarbon molecules, such materials make a suitable source for hydrocracking.
Vacuum Gas Oils: Vacuum gas oils derived from vacuum distillation of crude oil are another popular feedstock for hydrocracking. With high boiling points and heavier hydrocarbon compounds, they’re well suited to hydrogenation and cracking reactions inside a hydrocracker.
Residues: Residues from distillation can be utilized as feedstock for hydrocracking. Since these residues contain complex hydrocarbon molecules that require further processing into products of value, hydrocracking offers an effective means to upgrade these heavy fractions into lighter fractions that yield higher returns on investment.
Atmospheric Tower Bottoms: Heavy fractions from atmospheric distillation columns used during refining processes may serve as feedstock for hydrocracking, providing high molecular weight hydrocarbons that can be transformed into lighter products through hydrogenation and cracking processes.
Deasphalted Oils: Deasphalted oils are produced through a process known as deasphalting, in which asphaltic residues are removed from heavy crude oil or vacuum residue. As a result, deasphalted oils contain considerably less asphalt, making them suitable for hydrocracking production of lighter and cleaner products.
Note that selecting feedstock depends on a range of factors such as refinery configuration, desired product yields, market demands and specific objectives of hydrocracking unit. Refineries will select feedstocks that can be efficiently processed by their hydrocracker to produce high-quality fuels and petrochemical feedstocks while optimizing overall refining operations.
Comparison Table of Catalytic Cracking and Hydrocracking
Below is a comparison table highlighting the key differences between catalytic cracking and hydrocracking:
| Aspect | Catalytic Cracking | Hydrocracking |
|---|---|---|
| Objective | Conversion of heavy hydrocarbons to lighter products | Conversion of heavy hydrocarbons while hydrogenating them |
| Catalyst | Acidic materials (zeolites, silica-alumina, etc.) | Metal catalysts (platinum, palladium, etc.) |
| Feedstock | Heavy gas oils, vacuum residue, etc. | Heavy gas oils, vacuum gas oils, residues, etc. |
| Reaction Conditions | High temperature and low pressure | High temperature and high pressure |
| Product Yield | Higher yield of gasoline and olefins | Higher yield of diesel and lighter, sulfur-free fuels |
| Product Quality | Lower quality products (more olefins, less hydrogen) | Higher quality products (less sulfur, nitrogen, aromatics) |
| Environmental Impact | Moderate environmental impact (emissions, waste) | Lower environmental impact (reduced emissions, waste) |
| Energy Requirements | Lower energy requirements | Higher energy requirements |
| Application Areas | Gasoline production, petrochemical feedstocks | Diesel production, clean fuels, lubricant base stocks |
| Process Complexity | Relatively less complex | Relatively more complex |
| Catalyst Regeneration | Less frequent catalyst regeneration required | More frequent catalyst regeneration required |
Please note that this table provides a general overview, and specific conditions and outcomes may vary depending on the refinery configuration, feedstock quality, catalyst formulation, and other factors.
Environmental impact and sustainability considerations
Catalytic cracking and hydrocracking, like any refining process, have environmental and sustainability considerations that must be considered. Here are a few key points regarding their Environmental impacts and Sustainability concerns:
Environmental Impact: Emissions: Both Catalytic cracking and Hydrocracking Processes can result in Emissions of Greenhouse gases, volatile organic Compounds (VOCs), sulfur oxides (SOx), nitrogen oxides (NOx), and Particulate matter that Contributes to air pollution – as well as have detrimental impacts on human health and the environment. These Emissions contribute to air pollution with Potentially negative Consequences for health as well as for the Environment.
Waste Generation: Refining processes generate a variety of waste streams, such as spent catalysts and other byproducts, that require proper management and treatment in order to limit their environmental impact.
Refining processes such as catalytic cracking and hydrocracking require large volumes of water for cooling purposes and process requirements, so managing and optimizing its use to preserve available resources.
Energy Consumption: Both catalytic cracking and hydrocracking processes consume significant energy inputs for heating and maintaining process conditions, so energy efficiency measures and using cleaner sources may help lower environmental impacts associated with energy usage.
Considerations of Sustainability:
Carbon Footprint: The refining industry, including catalytic cracking and hydrocracking, has made significant strides to lower its carbon footprint. Implementation of carbon capture and storage (CCS) technologies, improving energy efficiency measures, and exploring alternative energy sources can all help lower greenhouse gas emissions while mitigating climate change impacts.
Feedstock Selection: Feedstock selection plays a vital role in refining processes. Utilizing renewable or low-carbon feedstocks like bio-based or waste material-derived materials can increase sustainability within the refining industry and contribute to its long-term viability.
Optimizing Catalysts: Optimizing catalysts used in catalytic cracking and hydrocracking processes can significantly enhance process efficiency, minimize waste generation, and lower environmental impact. Finding more sustainable catalyst materials remains an ongoing research area.
Compliance and Environmental Regulations: Maintaining sustainable refining operations relies heavily on adhering to environmental regulations and industry standards, such as emissions limits, waste management laws and other environmental requirements. Following them ensures that refining processes minimize their negative impact on the environment while protecting human health.
Sustainability and environmental responsibility have become more prevalent in the refining industry. Refineries are adopting strategies and technologies to decrease their carbon footprint, increase energy efficiency, and adopt environmentally responsible and responsible processes – creating more environmentally responsible refining processes overall.
Potential future developments and advancements in both processes
Future developments and advancements of catalytic cracking and hydrocracking processes could be driven by multiple factors, including environmental concerns, market demands, and technological innovations. Here are some potential future advancements for both processes:
Catalyst Research and Development: Research and development efforts continue to focus on finding more efficient and selective catalysts for catalytic cracking and hydrocracking processes, in order to enhance process performance, increase product yields, and enhance final product quality.
Process Integration: Refineries may look for ways to integrate catalytic cracking and hydrocracking processes with other refining units in order to increase operational efficiencies and maximize overall operational efficiencies. Integration may involve improved heat integration, feedstock pre-treatment or using byproducts from one process as feedstock for another process.
Renewable Feedstocks: With increased emphasis on sustainability and carbon emissions reduction, renewable feedstocks such as bio-based biomass or waste materials could become increasingly attractive as sources for catalytic cracking and hydrocracking processes. Such exploration could reduce reliance on fossil fuels while contributing to lower carbon footprint.
Process Flexibility: Refineries may focus on increasing the flexibility of their catalytic cracking and hydrocracking processes to accommodate for a wide variety of feedstocks, such as unconventional oils, heavy oils and alternative feedstocks. This enables refineries to adapt more readily to changing market demands while optimizing feedstock utilization.
Process Efficiency and Energy Optimization: Future advancements may include methods that aim to further maximize process efficiency while decreasing energy usage in catalytic cracking and hydrocracking processes, including advanced heat integration technologies, enhanced catalyst regeneration techniques, and the implementation of energy-saving technologies.
Environmental Sustainability: Steps are being taken to limit the environmental impacts associated with catalytic cracking and hydrocracking processes. These measures include carbon capture and storage technologies (CCS), use of renewable energy sources such as wind or solar, improved emission controls, waste management procedures, as well as advances in emission monitoring practices.
Digitalization and Automation: Leveraging digitalization, data analytics, and automation technologies to optimize process control can increase operational efficiency while improving product quality. Furthermore, advanced monitoring and control systems offer real-time insights for process optimization as well as predictive maintenance.
As stated previously, future developments will depend on many different factors including market dynamics, regulatory requirements, research advances and their economic viability. Still, the industry’s commitment to sustainability, efficiency and meeting evolving market needs will likely drive ongoing advances in catalytic cracking and hydrocracking processes.
Conclusion
Catalytic cracking and hydrocracking are two essential refining processes with distinct applications and characteristics. Catalytic cracking focuses on the conversion of heavy hydrocarbons to lighter products such as gasoline and olefins while hydrocracking utilizes hydrogenation and cracking technologies to upgrade heavy feedstocks into cleaner diesel and jet fuel fuel sources.
Both processes offer distinct advantages and disadvantages. Catalytic cracking offers higher gasoline yields and is capable of yielding valuable petrochemical feedstocks; however, diesel production may be restricted due to additional refining steps required for sulfur removal.