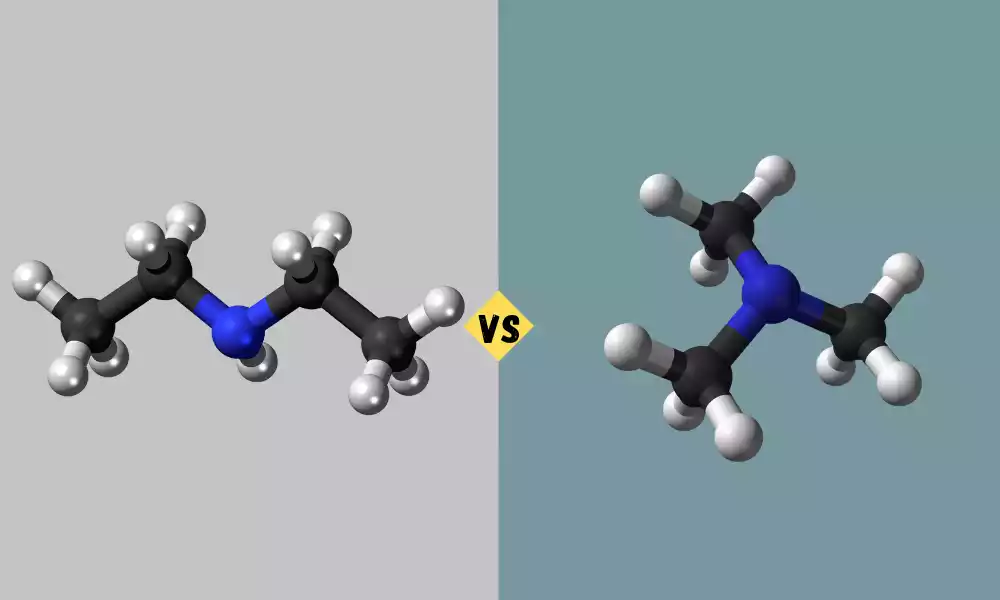
Dimethylamine and Trimethylamine are organic compounds with different chemical properties and structures. Dimethylamine (DMA) is composed of two methyl (CH3) groups that are attached to a nitrogen atom central as well and trimethylamine (TMA) is composed of three methyl groups linked to the nitrogen atom.
This results in a variety of chemical reactivity and application. DMA is an inert gas that has the odor of fish and is widely used for the manufacture of herbicides, pharmaceuticals, as well as rocket propellants.
TMA is a gas that has a strong fishy smell and is present in seafood, including fish, because of the microbial breakdown of trimethylamine oxide. Understanding the distinctions between these compounds is essential in determining their effectiveness in various industries from food production to agriculture chemicals and pharmaceuticals.
What is Dimethylamine?
Dimethylamine commonly referred to as DMA is a chemical compound that has its molecular formula (CH3)2NH. It is a tiny simple amine that is distinguished by the presence of two methacrylic (CH3) groups that are attached to a nitrogen atom in the central.
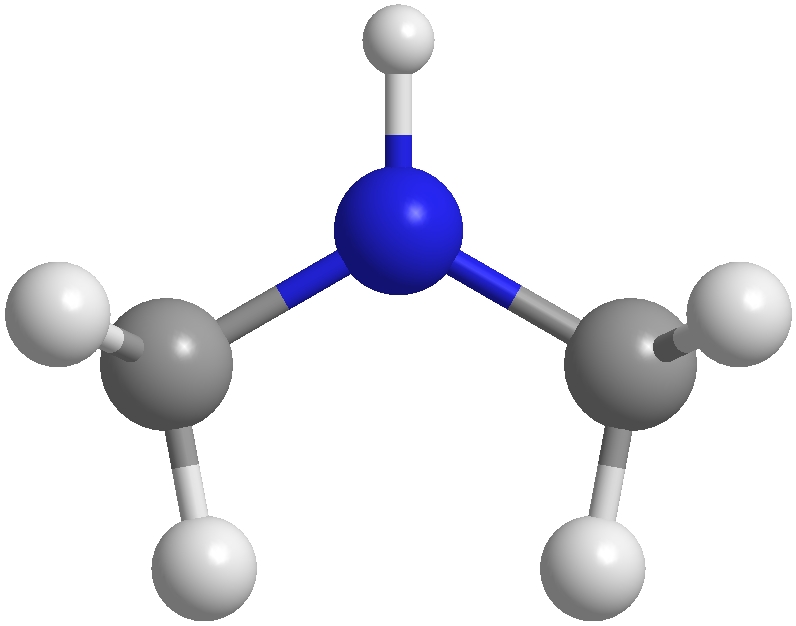
DMA is an inert gas that has an ammonia-like smell. It is highly soluble in water and is flammable when exposed to a flame. One of the most important applications of dimethylamine is its usage in the synthesis of chemical compounds in a variety of ways.
It is an important intermediate in the manufacture of herbicides, pharmaceuticals, and pesticides. DMA can also be used in the creation of rocket propellants where it is used as an element of fuel.
In the pharmaceutical sector, dimethylamine plays a significant role in the production of medicines such as ephedrine or metformin. It is also used in the creation of surfactants, dyes, and flocculants in the chemical sector.
Its flexibility and reactivity make it a vital element in many chemical processes, which contributes to its importance in organic chemistry.
What is Trimethylamine?
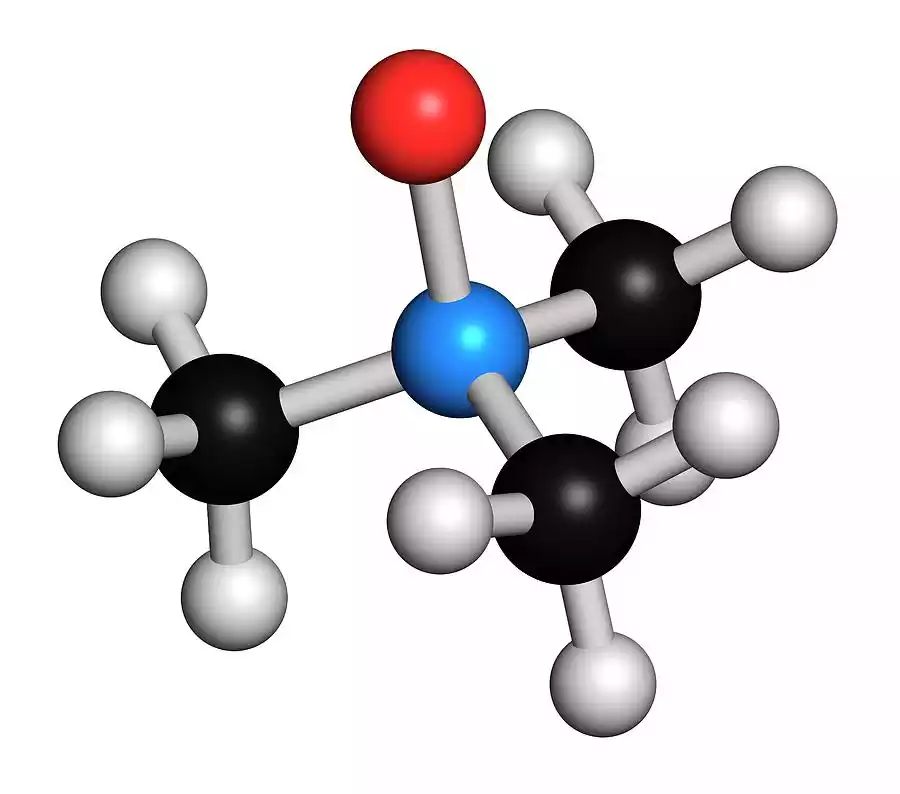
Trimethylamine, commonly abbreviated as TMA, is an organic substance that has a chemical formula (CH3)3N. It is a colorless and volatile gas that is distinguished by its distinct, pungent fishy smell, which can be found in seafood and decaying fish.
TMA is a tertiary chemical amine made up of three methyl (CH3) groups that are connected to a nitrogen atom in the central.
A single of the widely-known sources of trimethylamine is microbial degradation of trimethylamine oxide. This is found in fish as well as other seafood. This process is responsible for this “fishy” smell associated with spoilt seafood.
Trimethylamine is used in a variety of industrial applications. It is utilized as an element in the synthesis of various chemical compounds such as choline, which is a vital nutrient.
TMA is also used in the manufacture of quaternary ammonium compounds and is a crucial intermediate in the manufacture of specific pharmaceuticals, dyes also inhibitors. Its distinctive odor and function in life chemistry make trimethylamine a chemical with both biological and practical importance.
What is the Importance of Dimethylamine and Trimethylamine?
Trimethylamine and dimethylamine are significant substances in a variety of bio- and industrial settings:
- Chemical Synthesis: Dimethylamine as well as trimethylamine function as essential components for the synthesis of diverse chemicals. Dimethylamine is employed for the production of herbicides, pharmaceuticals, and rocket propellants. trimethylamine is used in the production of the quaternary ammonium compound, choline, and dyes, pharmaceuticals as well as corrosion inhibitors.
- Agriculture: Dimethylamine can be a key ingredient when it comes to the formula of herbicides that are employed to control crops and weeds while ensuring the productivity of crops.
- Pharmaceuticals: Dimethylamine can be a major intermediate within the industry of pharmaceuticals, assisting in the manufacturing of medicines such as metformin and ephedrine.
- Food Chemistry: Trimethylamine causes the odor of fish in fish and seafood. Knowing the role of trimethylamine in food chemistry is crucial to ensure food quality and security.
- Biochemical Processes: Trimethylamine can be produced naturally by microbial degradation in fish. It is a key component in the biological function of aquatic life. It also plays a role in the metabolism of choline in humans as well as other animals.
Properties of Dimethylamine and Trimethylamine
Dimethylamine (DMA):
- Chemical Formula: (CH3)2NH
- State at room temperature At room temperature, it is gaseous however it is able to be stored as a pressure-sensitive liquid.
- Odor The odor is a strong ammonia-like odor.
- Solubility Very soluble in water.
- Boiling Point: Approximately -6.2degC (-21.2degF).
- Melting Point: -92.2degC (-134.0degF).
- Density Around 0.68 g/cm3.
- Molar Mass Around 45.08 g/mol.
- Reactivity Utilized as a catalyst in the synthesis process of diverse chemicals, including herbicides and pharmaceuticals.
- Security: Flammable and potentially toxic if inhaled or absorbed into the skin.
Trimethylamine (TMA):
- Chemical Formula: (CH3)3N
- State at room temperature: It is a gas at room temperature and has a sour smell of fish.
- Odor: A distinctive strong smell of fish, which is often related to the decaying of seafood and fish.
- Solubility: Solubility is moderately water-soluble.
- Boiling Point: Approximately 2.9degC (37.2degF).
- Melting Point: -117.6degC (-179.7degF).
- Density: Around 0.7 g/cm3.
- Molar Mass: About 59.11 g/mol.
- Reactivity: It is an element for the synthesis of chemicals like choline and quaternary ammonium compounds.
- Safe: Can be hazardous if ingested or inhaled the odor, which is pungent, is a warning sign to avoid exposure.
How is Dimethylamine and Trimethylamine produced?
Production of Dimethylamine (DMA):
- Catalytic dehydration of Methanol: The most common method for making dimethylamine is through the catalytic dehydration process of Methanol (CH3OH) when it is in conjunction with a catalyst that dehydrates such as silica or alumina. This process creates dimethylamine with water as the byproduct. The reaction could be interpreted by the following:- 2 CH3OH – (CH3)2NH + H2O
- Amination of Methylamine: An alternative method to produce dimethylamine is the Amination of methylamine [(CH3)NH2] by formaldehyde [(CH2O)to produce dimethylamine. This method is not widely utilized, but it offers a different method to DMA.
Production of Trimethylamine (TMA):
- Methylation of Ammonia: Trimethylamine is usually made by the methylation from ammonia (NH3) by using formaldehyde or methanol as a catalyst for methylation. The reaction is able to be generalized in the following manner: – 3 CH3OH + NH3 – (CH3)3N + 3 H2O
- Catalytic Methylation: In certain cases ammonia reacts by methanol with the help of catalysts such as silica or alumina, to make trimethylamine as well as water.
Chemical Structures
Dimethylamine (DMA):
- Molecular Formula: (CH3)2NH
- Structural Formula: CH3-NH-CH3
Trimethylamine (TMA):
- Molecular Formula: (CH3)3N
- Structural Formula: CH3-N-CH3-CH3
Uses and Applications of Dimethylamine and Trimethylamine
Dimethylamine (DMA):
- Pharmaceuticals: DMA can be found to make pharmaceuticals like metformin and ephedrine. It’s a key intermediate in the creation of these drugs and others.
- Agriculture: DMA can be an essential ingredient when it comes to the formulation of herbicides. Herbicides that have DMA are employed in agriculture to manage the growth of weeds and increase yields.
- Rocket propellants: DMA can be used to make certain rocket propellants. It is an element of fuel.
- Chemical Synthesis: DMA is an element of construction in the manufacture of various chemicals. These include surfactants, dyes, as well as flocculants. It plays a role in a variety of chemical processes that are used in the industrial industry.
Trimethylamine (TMA):
- Food Chemistry: The TMA chemical is the cause of the odor of fish found in seafood and fish because of its presence in the decaying of fish. The detection of TMA is vital for the control of quality in the industry of seafood.
- Chemical Synthesis: It is utilized as a construction block for the synthesis of chemicals such as Choline, an essential nutrient, and also quaternary ammonium compounds, as well as chemical compounds that are of specialization.
- Corrosion inhibitors: TMA is utilized in the manufacture of corrosion inhibitors, substances that are used to protect metallic surfaces against corrosion.
- Pharmacies: It is involved in the production of certain pharmaceuticals, but its uses in this field are not as widespread as the ones of DMA.
Key Difference Between Dimethylamine and Trimethylamine
Here’s a comparison chart summarizing the key differences between Dimethylamine (DMA) and Trimethylamine (TMA):
| Property/Characteristic | Dimethylamine (DMA) | Trimethylamine (TMA) |
|---|---|---|
| Molecular Formula | (CH3)2NH | (CH3)3N |
| Chemical Structure | H-N-H | H-N |
| State at Room Temperature | Gaseous | Gaseous |
| Odor | Strong ammonia-like odor | Strong fishy odor |
| Solubility in Water | Highly soluble | Moderately soluble |
| Boiling Point | Approximately -6.2°C | Approximately 2.9°C |
| Melting Point | -92.2°C | -117.6°C |
| Density | Approximately 0.68 g/cm³ | Approximately 0.7 g/cm³ |
| Molar Mass | Approximately 45.08 g/mol | Approximately 59.11 g/mol |
| Environmental Impact | Potential contamination, relatively low persistence | Naturally occurring in fish and seafood, affects air quality |
| Health Concerns | Toxic upon inhalation, skin contact, and ingestion | Irritant to respiratory tract, eyes, and skin |
| Main Applications | Pharmaceuticals, herbicides, rocket propellants, chemical synthesis | Food chemistry, chemical synthesis, corrosion inhibitors |
Environmental and Health Considerations
Health and environmental considerations are essential to understanding the effects of dimethylamine (DMA) and trimethylamine (TMA) on the human environment and health.
Here are some of the most important aspects in relation to their effects on health and the environment:
Dimethylamine (DMA):
- Environmental impact: DMA may contribute to environmental pollution if it is not properly managed. The release of DMA into the water can have an impact on the aquatic ecosystem. It is, however, considered to be a fairly small environmental impact.
- Health Risks: DMA can be hazardous if it is inhaled or it is in contact with skin or eyes. Long-term exposure or consumption can cause respiratory irritation or skin burns as well as other health problems. Proper protective gear and handling methods are required in the case of DMA to limit the risks.
Trimethylamine (TMA):
- Environment Impact Natural TMA generated during the breakdown from organic matter could cause the distinctive smell of decomposing seafood and decaying fish. The release of it into the environment could affect the quality of air, especially in marine environments and fish processing facilities.
- Health Risks: Exposure to TMA could cause irritation to the eyes, respiratory tract, and skin. Even though TMA isn’t classified as extremely toxic, adequate protection and ventilation are advised when handling this chemical, particularly in industrial environments.
Toxicity and Safety Considerations
Here are some important information concerning their safety and toxicity:
Dimethylamine (DMA):
- Toxicity DMA could be toxic when breathed in, eaten or comes into contact with eyes and skin. It is an irritant to the respiratory system and could result in skin irritation if it is not treated properly. Long-term exposure could lead to health problems.
- Safety measures: In order to ensure your safety while working using DMA proper Personal Protective Equipment (PPE) must be worn with gloves, safety goggles or lab coats. DMA should only be used in a well-ventilated area and exposure levels must be monitored to avoid excessive exposure. Showers for emergencies and eye wash stations must be accessible in locations in which DMA is being used.
Trimethylamine (TMA):
- Toxicity The TMA compound, although not categorized as extremely toxic, can cause irritation to the eyes, respiratory tract and skin after exposure. It is crucial to avoid the inhalation of or direct contact with the substance.
- Safety measures: Security measures to protect TMA provide adequate ventilation in work areas to reduce exposure. Equipment for personal protection, like goggles, gloves, or lab coats must be worn. As with DMA, the emergency equipment such as eye wash stations, as well as showers for emergencies should be accessible in areas that handle TMA.
Summary
Trimethylamine is most often associated with the distinctive smell of rotting seafood and fish and is made by the microbial breakdown of trimethylamine oxide in these foods. Alongside its olfactory properties trimethylamine is a chemical building block that can be used for a range of industries.
It is employed for the manufacture of the quaternary ammonium compound, choline and pharmaceuticals, dyes along corrosion inhibitors. Its wide array of uses and unique function in both biological and industrial situations make trimethylamine an incredibly important compound.