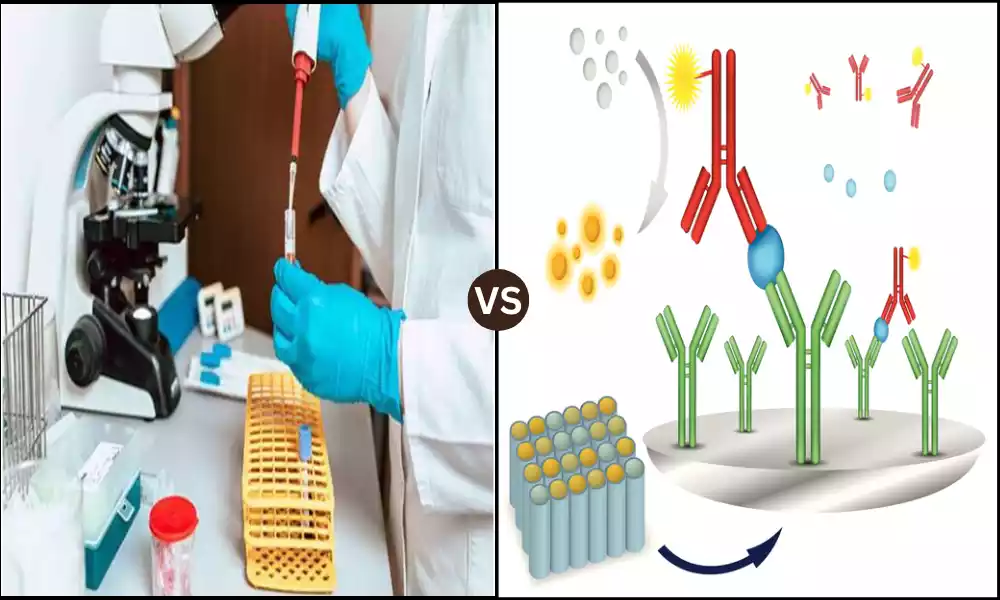
The enzyme-linked immunosorbent assays (ELISA) are the most important instruments for biomedical research as well as diagnostics, providing accurate analysis and detection of particular molecules.
Two main variations, Sandwich Elisa and Competitive Elisa are distinguished through their principles, uses, and their utility in a variety of applications in science. This article outlines the major differences between the two ELISA methods and sheds light on their specific techniques, target molecules, and benefits for an in-depth knowledge of their role in the field of diagnostics using immunology.
What is Sandwich Elisa?
The Sandwich ELISA (Enzyme-Linked Immunosorbent Assay) is an immunoassay that is used in biomedical research as well as diagnostics to identify and quantify the presence of targets (usually protein molecules or big molecules) within a biological sample. It’s called a “sandwich” assay because it requires the use of two antibodies that “sandwich” the target antigen of significance between them. This is a more precise definition:
In a Sandwich ELISA:
- Capture Antibody: The first antibody, referred to by the name of “capture antibody,” is coated or immobilized on the surface of a microplate. The antibody is extremely specific to the antigen of interest and helps to bind or capture the antigen if it’s found in the specimen.
- Sample addition A biological sample (e.g. plasma, serum, or even tissue extract) is added to the microplate with the capture antibody. If the antigen of interest is found in the sample it will bind the antibody that is captured, thereby attaching it to the well surface.
- Washing: Once you have allowed sufficient time for antigens to adhere to the antibody, unbound samples are cleaned to eliminate any binding that is not specific.
- Antibody to Detection: A second antibody, also known by the name of “detection antibody,” is added to the well. It is specific to the exact target antigen however it recognizes a different epitope (a different portion) that is part of the antigen in comparison to the capture antibody.
- Enzyme conjugate: In certain instances, the detection antibody can be conjugated to one of the enzymes (e.g. horseradish peroxidase or alanine peroxidase) to enhance the signal. The enzyme-conjugated detection antibody will connect to the antigen which already binds to the antibody that is being captured creating a “sandwich” structure.
- Substrate addition: Substrate Solution with a colorless, or faintly colorless substrate can be added to the. When the antibody used to detect is conjugated to enzymes this enzyme causes a chemical reaction in the substrate.
- Signal Measurement: The reaction that is enzymatic produces visible signals, which can be the appearance of a change in color or fluorescence. The intensity that the signals produce is proportional to the quantity of bound antigen, and consequently, the amount of the antigen target present in the test.
In the process of measuring the signal, and then comparing it with the standard curve that is generated from known concentrations of target antigen, researchers are able to accurately determine the amount of antigen present in the sample.
This is what makes Sandwich ELISA a valuable tool for determining the amount of specific proteins like biomarkers, and diverse biological samples for research or to aid in diagnosis.

What is Competitive Elisa?
The Competitive ELISA (Enzyme-Linked Insulant Assay) is a kind of immunoassay that is used in biomedical research and diagnostics. It is used to determine the presence and amount of target Analytes (such as antibodies and tiny molecules) in biological samples.
In a Competitive ELISA, The most significant characteristic is that the labeled antigen or molecule competes with the specific analyte in the sample to bind to a small amount of sites on a captured antibody. This is a more precise definition:
In a Competitive ELISA:
- Capture Antibody: Capture Antibody that is specific to the targeted analyte is immobilized or coated on the surface of a microplate. This antibody stays in the microplate throughout the test.
- Sample addition The biological sample that contains the analyte of your choice can be added into the well together with the known amount of labeled antigen molecules. The molecule that is labeled is identical or like the analyte in regards to its ability to connect to the antibody that is captured.
- Competition for binding sites The antigen labeled and the analyte contained in the sample are competing for binding sites that are available on the antibody that is captured. The amount of their binding depends on their respective concentrations within the sample.
- Washing: Once the time has been allowed for binding to take place, the molecules that are not bound are removed by several washing steps. This leaves only the ones that have successfully been bound to the antibody on the surface of the well.
- Substrate addition: Substrate Solution usually contains the colorless or faintly colored substrate that is introduced into the well. If the antigen labeled is conjugated with enzymes, it could cause a chemical reaction to the substrate.
- Signal Measurement: The reaction causes a discernable signal, usually an increase in color or fluorescence. The intensity of this signal is proportional to the amount of the analyte within the sample. That is a greater amount of analyte causes lesser binding for the antigen labeled and, as a result, an increased signal however, a lower amount of analyte results in greater binding of the antigen labeled and weaker signal.
By taking the signal and comparison to the standard curve that is generated from known concentrations of analytes researchers can calculate the amount of the analyte present in the test. Competitive ELISA is used extensively to detect and quantify small molecules, hormones, chemicals, or antibodies in a variety of clinical and biological samples.
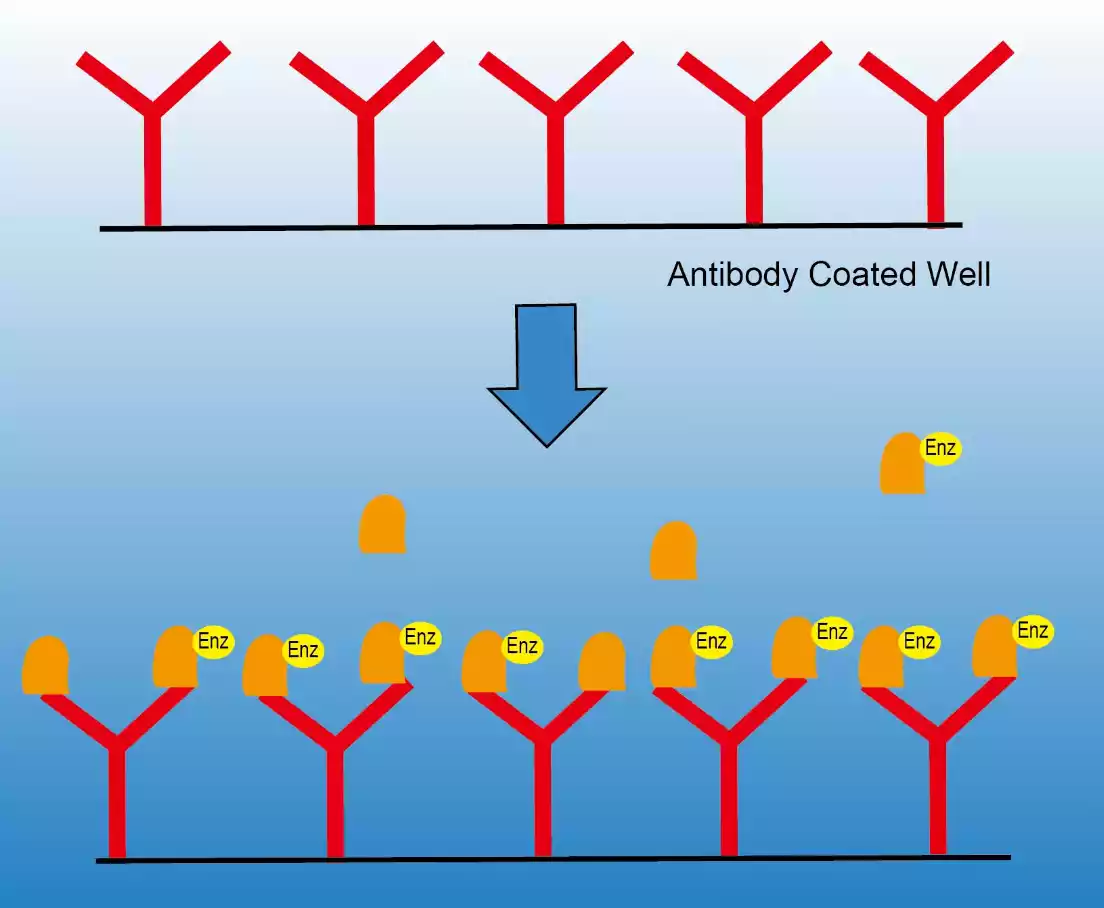
Importance of ELISA in biomedical research and diagnostics
ELISA (Enzyme-Linked Inspiring Assay) is a key instrument in biomedical research and diagnostics because of its sensitivity, versatility, and specificity.
Its significance in these fields can be described as follows:
- Disease Diagnosis:
- Infectious Diseases: ELISA is extensively used to identify antibodies or antigens linked to diseases that are infectious, like HIV or hepatitis. COVID-19. It allows for the early detection and monitoring of these diseases.
- Autoimmune diseases: ELISA assists in the diagnosis of autoimmune diseases such as rheumatoid arthritis or systemic lupus, by detecting autoantibodies.
- Cancer: It assists in the diagnosis of cancer by analyzing the markers of cancer within blood specimens, assisting with the early identification of cancer, and monitoring the effectiveness of treatment.
- Monitoring and Management:
- Hormone measurement: ELISA is crucial in assessing the levels of hormones (e.g. thyroid hormones, insulin) for thyroid and diabetes issues, and helping to guide the treatment changes.
- Monitoring Drugs: It assists in monitoring the concentration of drugs (e.g. therapeutic medications, drugs of abuse) in patients in order to provide effective treatment and to prevent toxicity.
- Test for Allergies: The ELISA test is used to determine allergens that cause allergic reactions. This allows for the development of customized treatment strategies.
- Vaccine Development:
- ELISA is a crucial part of developing vaccines through the measurement of the specific antibodies that are produced during vaccination, to ensure the safety and effectiveness of vaccines.
- Research Tools:
- ELISA is utilized in research fundamentally to study the interactions between proteins and proteins, signal transduction pathways, and gene expression by the quantification of biomolecules specific to a particular species.
- It assists in the development of biomarkers that are new to identify ailments, which contribute to a better understanding of the mechanisms of disease.
- Quality Control in Biotechnology:
- ELISA can be used to test the purity and quality of biopharmaceutical substances, such as monoclonal antibodies as well as recombinant proteins as well as to ensure safety and consistency.
- Food and Environmental Analysis:
- For food safety, ELISA detects contaminants such as allergens or pathogens as well as toxic substances, which ensure the food safety of all products.
- ELISA is also utilized in environmental monitoring to identify pesticides, pollutants, and toxins in the air, water as well and soil samples.
- High-Throughput Screening:
- With the help of automation, ELISA can process large quantities of samples at once and is therefore effective for screening with high throughput for drug discovery and clinical labs.
- Point-of-Care Testing:
- Point-of-care tests using ELISA offer quick results for medically critical decision-making in settings with limited resources which improve access to healthcare.
- Specificity and Sensitivity:
- ELISA provides high sensitivity, with a low number of false positive results, and has a high sensitivity, which allows the identification of low levels of analytes.
- Clinical Trials:
- ELISA can be used to evaluate the pharmacokinetics as well as pharmacodynamics of drugs in clinical trials. It aids in the development of drugs and ensures their approval.
ELISA is an indispensable instrument in biomedical research and diagnostics. It is a significant factor in diagnosis, monitoring, treatment, and improving our understanding of a myriad of health-related issues. Its flexibility and accuracy make it a crucial element of modern healthcare and research in science.
Comparison Table of Sandwich Elisa and Competitive Elisa
Here’s a comparison table highlighting the key differences between Sandwich ELISA and Competitive ELISA:
| Aspect | Sandwich ELISA | Competitive ELISA |
|---|---|---|
| Target Molecule | Antigens | Antibodies, small molecules |
| Antibody Configuration | Two antibodies (capture and detection) | One antibody (capture) and labeled antigen/molecule |
| Signal Relationship | The direct relationship between signal and antigen concentration | The inverse relationship between signal and analyte concentration |
| Purpose | Quantification of target antigen | Detection of antibodies or small molecules |
| Typical Applications | Biomarker quantification, disease diagnosis, drug development | Antibody detection (e.g., HIV antibodies), small molecule detection (e.g., hormones), immunoassays |
| Sensitivity and Specificity | High sensitivity and specificity | High sensitivity and specificity |
| Complexity | Requires two specific antibodies, complex assay setup | Simpler setup compared to Sandwich ELISA |
| Quantification | Suitable for quantification | Less quantitative, often used for qualitative detection |
| Multiplexing | Multiplexing capability for detecting multiple antigens | Limited multiplexing capability |
| Common Use Cases | Protein quantification, cytokine detection | Antibody titer determination, small molecule screening |
This table summarizes the primary distinctions between Sandwich ELISA and Competitive ELISA, including their target molecules, antibody configurations, signal relationships, purposes, applications, complexity, quantification capabilities, and common use cases. Each ELISA type has its unique strengths and is chosen based on the specific research or diagnostic needs.
Substrate addition and signal measurement
Both Sandwich ELISA, as well as Competitive ELISA, substrate addition, and signal measurement, are essential methods for determining that the sample contains the targeted analyte. This is how the steps usually are carried out in each assay
Substrate Addition and Signal Measurement in Sandwich ELISA:
- Substrate Addition:
- Following the detection antibody, enzyme conjugate, and washing processes, a substrate solution will be added to the wells of the microplate.
- The typical substrate is either colorless or a faintly colored solution.
- Enzymatic Reaction:
- When the protein (conjugated to the detected antibody) binds on the surface of the plate because of being able to recognize the antigen targeted It initiates chemical reactions to the substrate.
- This reaction causes the generation of a visible signal, which is usually a color product.
- Signal Measurement:
- The color development on the surface of wells is directly related to the enzyme amount present which in turn is dependent on the amount of the target antigen present in the sample.
- The absorbance, also known as optical density (OD) of the color product is measured with the microplate reader at a certain wavelength (e.g. at 450 nm).
- The higher the amount of antigen greater the signals (higher Od).
Substrate Addition and Signal Measurement in Competitive ELISA:
- Substrate Addition:
- As with Sandwich ELISA After the washing process, the substrate solution will be added to the microplates.
- Enzymatic Reaction:
- In Competitive ELISA the amount of antigen that is bound to the capture antibody is in inverse proportion to the amount of analyte present in the sample.
- When the antigen that is labeled is bound to the antibody, it battles the analyte present in this sample to find binding spots. Therefore, the amount of antigen labeled bound is inversely proportional to the concentration of the analyte.
- Signal Measurement:
- The degree of enzymatic reaction (color change) during Competitive ELISA is in inverse correlation in relation to the level of analyte.
- The lower the concentration of the analyte the greater amount of labeled antigen is attached to the antibody to capture, which leads to greater enzyme activity and higher OD.
- A greater concentration of analytes results in lower binding of antigens labeled a decrease in enzyme activity and a lesser OD.
Although each Sandwich ELISA and Competitive ELISA include substrate addition and measurement of signals, the nature of the signal-to-substance relationship is different between the two methods. For Sandwich ELISA, the signal is directly proportional to antigen concentration.
However, in Competitive ELISA the signal is in inverse proportion to the concentration of analytes. These distinctions are essential to determining the presence and concentration of the target molecules in each test.
The inverse relationship between signal and antigen concentration
The inverted relationship between the signals and the concentration of antigens for Competitive ELISA is an essential idea that is the basis of the principle behind the test. This relationship is inverted because competitive ELISA is intended to quantify the competitiveness between an antigen that is labeled (often coupled by some enzyme) and the analyte (antigen of significance) within the sample for binding to a specific quantity of binding spots on a captured antibody.
This is how the reverse relationship is constructed:
- low antigen concentration (High competition from Antigens Labeled):
- If the concentration of analytes within the specimen is small, there is little potential for binding site competition on the antibody.
- In the end, more of the antigen labeled (conjugated by the enzyme) is bound to the capture antibody since there are fewer analyte molecules to fight.
- The bound enzyme is responsible for the transformation of the substrate into a visible product at a greater rate, resulting in an increase in sign (higher optical density, also known as OD).
- Very High Concentration of Antigen (Low Competition from Antigens that are Labeled):
- However, when the concentration of analytes in the test sample is extremely high it is very competitive for binding sites for the capture antibody.
- A greater number of analyte molecules are able to bind to the antibody, which will re-displace the antigen labeled.
- In the end, fewer antigens with labels will bind to the antigens in the capture antibody, resulting in a less active enzyme and a less effective sign (lower the OD).
In Competitive ELISA The quantity of the signal produced (e.g. the development of color) is in indirect proportion to the amount of analyte present in the sample. This inverted relationship is an essential element of the Competitive ELISA quantitative method.
By determining the intensity of the signal and then comparing it with the standard curve or standards with analyte concentrations that are known Researchers can precisely determine the amount of analyte present in the sample, even if it’s only present at very low levels.
Adding sample antigen and labeled antigen
When performing the case of an ELISA (Enzyme-Linked immunosorbent assay) adding the sample antigen along with the labeled antigen is an essential process in each Sandwich ELISA and Competitive ELISA however the manner in which these components are added as well as how they interact with the assay components varies between the two types of essays:
- Sandwich ELISA:
Adding Sample Antigen:
- When performing Sandwich ELISA, the sample with the antigen that is of significance is inserted into microplate wells, which were previously coated with the capture antibody.
- The antibody to be captured binds the antigen that is present in the sample, thereby securing it on the surface of the well.
Adding Labeled Detection Antibody (or Enzyme Conjugate):
- After washing off unbound sample components, a dyed anti-detection antibody (or one that is an enzyme conjugate) is added to the wells.
- It is specialized to a distinct epitope of the antigen in comparison to the antibody used for capture and therefore recognizes another region that is the exact antigen.
- The detection antibody labeled with a specific marker is able to bind to the antigen that was captured and forms a “sandwich” structure. If you use an enzyme conjugate it results in the antigen being labeled with an enzyme.
- Competitive ELISA:
Adding Sample Antigen and Labeled Antigen:
- In Competitive ELISA The sample that contains the analyte (e.g. antibodies, antibodies, or small molecules) as well as a labeled antigen (or the molecule that is labeled) are simultaneously added onto the microplates.
- The antigen labeled (or labeled molecules) is a rival and competes with the analyte within this sample to find binding points to the capture antibody.
Competition for Binding Sites:
- The extent to which the antigen labeled and the analyte are bound to the antibody that is captured can be determined from their respective concentrations.
- If the analyte is plentiful present in the samples, then it is likely to be able to outcompete the antigen that is labeled to be able to bind to the antibody that is captured. This means that fewer antigens that are labeled will be able to bind.
- If the analyte is not present within the specimen, then more labeled antigens will bind to the antibody that is used to capture.
In Competitive ELISA the signal produced (e.g. the color of development) is in inverse proportion to the amount of analyte within the sample. The amount of bound-labeled antigen is proportional to the concentration of analyte and results in a lower signal when the substance is plentiful and a higher signal when it is in a low supply.
Even though the two Sandwich, as well as Competitive ELISAs, include the samples and assay components and assay components, their interactions with the antibody that captures them and then the subsequent generation of signals differ significantly, resulting in different methods of quantification.
Suitable for detecting antibodies or small molecules
The decision between Sandwich ELISA and Competitive ELISA is based on the type of analyte you’re trying to test for. This is the breakdown of which test can be used to detect small molecules or antibodies.
Suitable for Detecting Antibodies:
- Sandwich ELISA: is the preferred option for the detection of antibodies. Here’s why:
- For Sandwich ELISA, two antibodies are employed – an antibody to capture and an anti-detection antibody. The antibody used to capture specifically targets the antigen targeted (often the antibody) while the detection antibody binds an epitope different from that same antibody.
- The dual-antibody strategy gives high specificity to the target antibody, which makes it ideal for detection of antibodies.
- Some examples of the detection of antibodies by using Sandwich ELISA include HIV antibody testing as well as the detection of antibodies to autoimmune diseases.
Suitable for Detecting Small Molecules:
- Competitive ELISA: It is commonly used to detect small molecules. Here’s why:
- In Competitive ELISA The analyte (small molecules) competes with an antigen that is labeled (small molecules) in order to attach itself to a small number of sites for binding on an antibody.
- The amount of antigen labeled that is bound to the cap antibody is in direct proportion to the amount of analyte present in the sample.
- Competitive ELISA is especially useful for determining small molecules such as hormones, drugs, or toxic substances.
If you need to determine the presence of antibodies Sandwich ELISA is the most preferred option because of its dual-antibody method and its high specificity. However, when you want to identify smaller molecules Competitive ELISA will be the better choice since it is based on the competition between the labeled small molecule and the analyte in the interest. The selection between these two kinds of tests is based on the specifics of the targeted molecule and the degree of particularity needed for the test.
Reference Books
Certainly, here are some reference books in different fields of study that are highly regarded and cover a wide range of topics:
1. Science and General Knowledge:
- “A Short History of Nearly Everything” by Bill Bryson
- “Sapiens: A Brief History of Humankind” by Yuval Noah Harari
- “Cosmos” by Carl Sagan
2. Biology:
- “Biology” by Neil A. Campbell and Jane B. Reece
- “Molecular Biology of the Cell” by Bruce Alberts et al.
- “The Selfish Gene” by Richard Dawkins
3. Chemistry:
- “Chemistry: The Central Science” by Theodore L. Brown, H. Eugene LeMay, and Bruce E. Bursten
- “Organic Chemistry” by Paula Y. Bruice
- “Physical Chemistry” by Peter Atkins and Julio de Paula
4. Physics:
- “The Feynman Lectures on Physics” by Richard P. Feynman
- “Principles of Physics” by Resnick, Halliday, and Walker
- “A Brief History of Time” by Stephen Hawking
5. Mathematics:
- “Principles of Mathematical Analysis” by Walter Rudin
- “Linear Algebra Done Right” by Sheldon Axler
- “Introduction to Probability” by Joseph K. Blitzstein and Jessica Hwang
Conclusion
The knowledge universe is vast and diverse and these reference books provide only a small portion of the vast amount of information accessible across various disciplines of study. No matter if you’re interested in math, science history, or any other field the books provide useful knowledge and provide an excellent point of entry to explore.
Keep in mind that learning is a continuous adventure and the pursuit of knowledge is both stimulating and rewarding. Keep studying, reading, and broadening your horizons in order to gain greater knowledge of the world that surrounds you.