Introduction
Understanding the difference between electrolytes and nonelectrolytes is vital in many scientific and practical contexts. Electrolytes and nonelectrolytes are two distinct classes of substances which behave differently when dispersed into solutions or molten states.
Electrolytes are substances that dissolve to release charged particles capable of conducting electric current. Electrolytes play a critical role in Biological systems by Maintaining fluid balance, Transmitting nerve impulses, and supporting Essential Physiological processes.
On the other hand, nonelectrolytes do not dissociate into ions when dissolved and do not conduct electricity – instead remaining intact molecules in solution and impacting both its physical and chemical properties.
Characteristics and roles of electrolytes and nonelectrolytes, with particular attention paid to differences between them as regards behavior in solution, conductivity and their presence within bodily fluids, physiological processes as well as examples and applications that highlight their significance across different fields.
By Developing an in-depth knowledge of Electrolytes and nonelectrolytes, readers will Develop a greater Comprehension of their unique Properties, functions, and Implications in fields like chemistry, biology, medicine, and Everyday life.
Definition of electrolytes
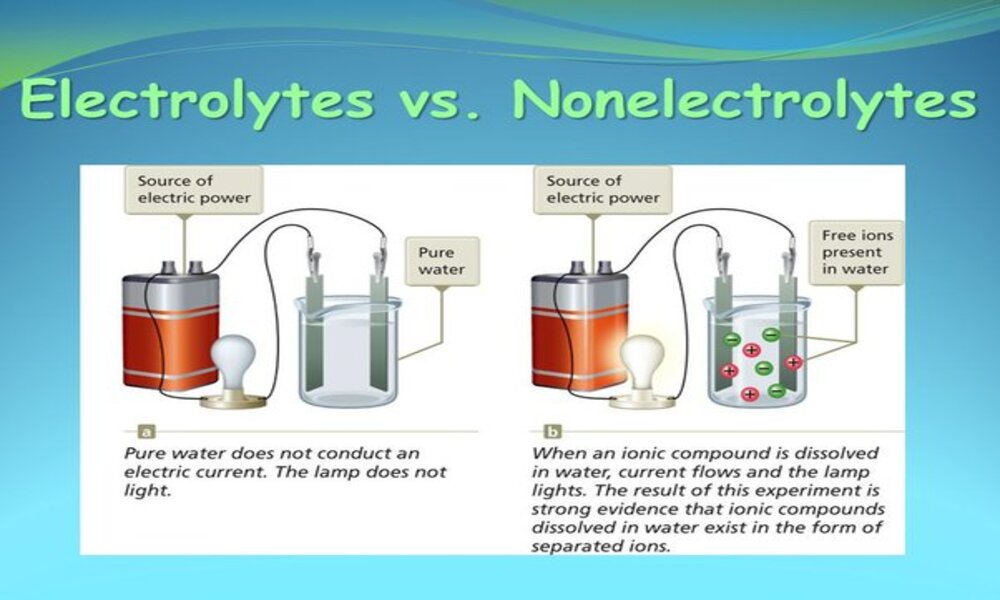
Electrolytes are substances that, when dissolved or in molten form, form ions capable of conducting electric current. Simply stated, electrolytes are compounds which dissociate into ions when in solution or liquid state.
These positively charged (cations) and negatively charged (anions) ions are responsible for electrolyte conductivity, and their presence enables electricity to flow more freely through them allowing electricity transmission.
Electrolytes, Commonly referred to as salts, acids, and bases, play an essential role in Biological systems for Maintaining fluid balance, Transmitting nerve impulses, and supporting various Physiological processes.Examples include sodium, potassium, calcium and chloride – each playing their own Important roles within biological Environments.

Types of electrolytes
There are two general classes of electrolytes, strong electrolytes and weak electrolytes.
Strong Electrolytes: Strong electrolytes are substances that dissociate into individual ions when dispersed into water or molten state solutions, producing an impressive degree of ionization resulting in large numbers of ions in solution and serving as excellent conductors of electric current.
Strong electrolytes include:
Strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4) and nitric acid (HNO3). Strong bases include sodium hydroxide (NaOH), potassium hydroxide (KOH) as well as strong salts such as sodium chloride (NaCl), potassium iodide (KI) and calcium chloride (CaCl2).
Weak Electrolytes: When dissolving in water or molten state, weak electrolytes only partially dissociate into ions; their degree of ionization is less compared to strong electrolytes and thus they have lower conductivities due to lower concentrations of ions present in solution and therefore reduced conductivity.
Examples of weak electrolytes include:
Weak acids include: Acetic acid (CH3COOH), carbonic acid (H2CO3).
Weak bases include ammonia (NH3).Salts commonly used as strong or weak electrolytes include ammonium acetate (NH4CH3COO) and copper sulfate (CuSO4), among many others. To make matters even more confusing, the classification of any given substance as either strong or weak electrolyte depends on how strongly its solution ionized is exhibited by that specific element.
Complete dissociation in solution
Complete dissociation in solution refers to the process whereby an electrolyte substance, typically water-based, fully dissociates into its constituent ions when dispersed into solution.Each molecule of this material dissociates fully into individual ions forming an abundant concentration of them within a solution.
Strong electrolytes typically undergo complete dissociation, in which nearly all dissolved substances dissociate into ions. This creates a solution with high conductivity due to numerous ions allowing efficient electric charge transference.
Table salt (NaCl) dissolves in water into sodium ions (Na+) and chloride ions (Cl-). Each NaCl molecule breaks up into one Na+ and one Cl- ion, yielding a solution with high concentrations of these two ions.
Dissociation of strong electrolytes in solution is typically driven by strong electrostatic forces between charged particles (ions) and polar solvent molecules, such as water molecules, which overcome intermolecular forces within the electrolyte and cause it to disperse into individual ions.
Not all electrolytes display complete dissociation in solution; weak electrolytes, for instance, only partially dissociate in water solutions compared with strong ones, leading to reduced concentrations of ions than when exposed to strong ones.
Conductivity of strong electrolytes
Strong electrolytes exhibit high conductivity in solution due to their ability to dissociate into individual ions easily, producing highly conducting solutions when mixed with water or another solvent.Strong electrolytes exhibit high conductivity due to the presence of many freely moving ions within their solution. These charged particles facilitate electric current flow by moving freely throughout its path through solution.
Due to strong electrolytes fully dissociating into ions, their concentration in solution is extremely high. This allows more charged particles available for electric current conductance.When an electric potential is applied across a solution containing strong electrolytes, positively charged ions (cations) move towards the negatively charged electrode (cathode), while negatively charged anions (anions) head towards the positively charged electrode (anode).
This phenomenon, known as ionic conductivity, allows electric current to flow freely throughout the solution.Strong electrolytes’ high conductivity results from their complete dissociation into ions, increasing concentration of charged particles in solution and allowing efficient movement of these ions – and hence electricity conduction.
Definition of nonelectrolytes
Nonelectrolytes are substances that do not dissociate into ions when dissolved in solution or molten state, unlike electrolytes that dissociate into free moving ions and conduct electricity. Nonelectrolytes exist as molecules both solid and in solution states.Common examples of nonelectrolytes include sugars (such as glucose and sucrose), alcohols such as ethanol and methanol, and organic compounds.
Nonelectrolytes dissolve in solvents without breaking apart into charged particles, so their molecules remain undisrupted and do not contribute to electrical conductivity.Although nonelectrolytes don’t contribute directly to electrical conductivity, their presence can still alter physical and chemical properties of the solvent such as boiling point elevation or freezing point depression.

Solubility of nonelectrolytes
Solubility of nonelectrolytes depends on their chemical compound and solvent of choice, with nonelectrolytes tending to exhibit different solubility characteristics than electrolytes.
Polar Nonelectrolytes: Many nonelectrolyte compounds, including sugars and alcohols, tend to dissolve readily in water due to favorable interactions such as hydrogen bonding or dipole-dipole interactions between their molecules and polar solvents like water. Examples include glucose, sucrose, ethanol and methanol.
Nonpolar nonelectrolytes: Nonpolar nonelectrolytes such as hydrocarbons tend to be insoluble in polar solvents like water due to being nonpolar substances which cannot form strong interactions with them.Instead, nonpolar nonelectrolytes tend to dissolve in nonpolar organic solvents like benzene or hexane – this includes fats, oils and many organic compounds with long hydrocarbon chains such as fats or oils.
Solubility can be affected by factors like temperature, pressure and other solutes present; nonelectrolytes may exhibit limited solubility in specific solvents leading to partial dissolution or suspensions rather than true solutions.The solubility of nonelectrolytes depends on their nature and compatibility with the solvent in which they’re being dissolved.
Conductivity of nonelectrolytes
Nonelectrolytes do not conduct electricity because their solution does not dissociate into individual ions upon dissolution, which allows conductivity in solutions to be maintained through charged particle movement (ions). Therefore, in nonelectrolyte solutions there is no electrical conductivity.
Nonelectrolytes dissolve into solvents as whole molecules that do not carry electric charges and cannot conduct electricity, meaning nonelectrolyte-rich solutions cannot allow electric current to pass through them.Nonelectrolytes lack conductivity due to their molecular composition and absence of ionization, unlike electrolytes which possess charged particles that transport electricity across solutions.
Note that nonelectrolyte solutions don’t conduct electricity; they still possess important physical and chemical properties that allow them to play various roles across industries and applications.
Comparison Table of Electrolytes and Nonelectrolytes
Here’s a comparison table highlighting the key differences between electrolytes and nonelectrolytes:
| Characteristic | Electrolytes | Nonelectrolytes |
|---|---|---|
| Dissociation in solution Conductivity | Fully dissociate into ions Conduct electricity due to freely moving ions. | Do not dissociate into ions, remain as intact molecules Do not conduct electricity. |
| Examples | Salts, acids, bases | Sugars, alcohols, organic compounds |
| Solubility | Generally highly soluble in water | Vary in solubility |
| Presence in bodily fluids | Found in bodily fluids (e.g., blood, sweat) | May or may not be present in bodily fluids |
| Role in physiological processes | Essential for nerve transmission, fluid balance, muscle contraction | Do not directly participate in physiological processes |
This table provides a concise overview of the key distinctions between electrolytes and nonelectrolytes.It highlights their behavior in solution, conductivity, examples, solubility, presence in bodily fluids, and their respective roles in physiological processes.
Electrolyte solutions in medical settings
Electrolyte solutions play an essential role in medical settings to restore and maintain body electrolyte balance, whether through oral, intravenous, or other means.Electrolyte solutions may be administered orally, intravenously, or through other routes to address electrolyte imbalances caused by dehydration, electrolyte losses, fluid shifts or certain treatments such as cancer treatments.
Below are examples of electrolyte solutions used in healthcare environments:
Oral Rehydration Solutions: These oral solutions are widely used to treat dehydration caused by conditions like diarrhea, vomiting or excessive sweating.Their formulation contains an equilibrium mixture of electrolytes including sodium, potassium chloride and sometimes glucose to replenish fluid and electrolyte reserves in the body.
Intravenous (IV) electrolyte solutions: Intravenous electrolyte solutions are administered directly into the bloodstream for rapid correction of severe electrolyte imbalances or fluid losses, and tailored specifically to individual electrolyte needs or combinations thereof. Common IV electrolyte solutions include:
Normal Saline (0.9% Sodium Chloride): This solution, composed of equal concentrations of sodium and chloride ions, can be used to provide fluid resuscitation, intravascular volume expansion and correct sodium imbalances.
Lactated Ringer’s solution: This solution contains electrolytes such as sodium, potassium, chloride calcium lactate for fluid replacement or correcting electrolyte imbalances. It is often used for fluid replacement purposes or correcting imbalances among electrolytes.
Potassium chloride solutions: Potassium chloride solutions provide an abundant source of potassium that can help treat or prevent low potassium levels (hypokalemia).
Magnesium Sulfate Solution: Magnesium sulfate solutions can be used to treat magnesium deficiency (hypomagnesemia). When necessary, this intravenous injection may be given in severe cases.
Dialysis Solutions: Dialysis solutions, also known as dialysate, are used in renal replacement therapy to remove waste products and excess fluids from the body through dialysis treatment. Designed to mimic natural electrolyte balance during this process, dialysate solutions contain specific electrolyte compositions and concentrations designed to maintain equilibrium for maximum effectiveness during dialysis therapy.
These are examples of electrolyte solutions used in medical settings. Their specific composition depends on each patient’s condition, electrolyte imbalances, and treatment objectives. Medical professionals closely monitor electrolyte levels to restore balance and facilitate proper physiological processes within their bodies.
Nonelectrolytes may or may not be present in bodily fluids
Nonessential electrolytes may or may not be present in bodily fluids. Blood, urine and interstitial fluid contain an array of substances including both electrolytes and nonelectrolytes.Electrolytes, substances that dissociate into ions when in solution, play an essential role in physiological processes within the body. Electrolytes help balance fluids, transmit nerve impulses and facilitate muscle contractions – common electrolytes found in bodily fluids are sodium, potassium, calcium, chloride bicarbonate phosphate.
On the other hand, nonelectrolytes – substances which do not dissociate into ions when dissolved – can also be present in bodily fluids. Examples include glucose, urea, creatinine, lipids and various organic compounds found in body fluids as nonelectrolytes; nonelectrolytes serve various functions within our bodies such as providing energy, being building blocks for cells or contributing to metabolic processes.
Body fluids containing nonelectrolytes do not contribute to their electrical conductivity; rather, their concentration and balance play an integral role in maintaining overall fluid composition and homeostasis. Electrolyte levels must be carefully managed for optimal physiological functioning.
Electrolytes play essential roles in various physiological processes
Electrolytes play an indispensable role in human physiological processes, from maintaining fluid balance to aiding nerve impulse transmission, supporting muscle contractions and contributing to various biochemical reactions.
Here are a few essential physiological processes where electrolytes play a critical role:
Electrolytes such as sodium, potassium and chloride help restore fluid equilibrium within and outside cells by creating an osmotic pressure that preserves optimal hydration levels, protecting from dehydration as well as overhydration.
Nerve Transmission: Electrolytes play a key role in transmitting nerve impulses. For example, sodium and potassium ions play an integral part in creating action potentials on nerve cells and spreading them along nerve bundles to create electrical gradients to carry signals between cells.
Muscle Contractions: Electrolytes like calcium, sodium and potassium play an integral part in muscle contractions. Calcium ions are necessary for cell contraction while sodium and potassium ions regulate action potentials that trigger contractions.
Acid-Base Balance: Electrolytes such as bicarbonate are integral in helping the body achieve the ideal acid-base balance, serving as buffers that keep its pH within an ideal range that supports enzyme functions and overall cell activities.
Kidney Function: Electrolytes play an essential part in maintaining fluid and electrolyte balance for kidney health, with kidneys helping maintain this by either reabsorbing them back into their system or expelling them through urine output.
Heart Function: Electrolyte imbalances between potassium and calcium ions may interfere with the normal electrical activity of your heart, potentially leading to arrhythmias or other cardiovascular issues. For optimal heart rhythm and health, adequate electrolyte levels must be maintained at all times.
Electrolytes play an integral part in many physiological processes within your body. Maintaining equilibrium among your electrolytes is key for overall wellness and smooth operation of bodily systems.
Nonelectrolytes do not participate directly in physiological processes
Nonelectrolytes do not directly contribute to physiological processes in the same manner that electrolytes do. Nonelectrolytes are substances that do not dissociate into ions when dissolved in solution and, thus, do not contribute to electrical conductivity.
Although nonelectrolytes may still play important functions within bodily fluids and play other vital roles within body systems like nerve transmission, muscle contraction or maintaining fluid balance, none have direct roles to play when it comes to physiological processes like nerve transmission, muscle contraction or maintaining fluid equilibrium.
Instead, nonelectrolytes typically play indirect roles in physiological processes; for example:
Energy Sources: Nonelectrolytes such as glucose and lipids provide energy sources to the body. While glucose from carbohydrates serves as primary fuel for cell metabolism, stored and utilized as reserves by fat cells as energy reserves.
Structural Components: Amino acids are the basic building blocks of proteins, which play an essential role in our body’s structural and functional needs. Nucleotides also comprise non-electrolytes but act as the building blocks for DNA and RNA synthesis.
Metabolic Reactions: Nonelectrolytes play an integral part in various metabolic reactions. For instance, nonelectrolytes can play an essential part in excreting waste products of protein metabolism such as urea from urine excreted by kidneys while creatinine used as a marker of kidney health can serve as an indicator.
Nonelectrolytes may not directly impact electrical signaling or fluid balance like electrolytes do; nonetheless, they still play important roles in cell function, metabolism and body composition. Both electrolytes and nonelectrolytes must coexist for proper physiological functioning.
Conclusion
Electrolytes and nonelectrolytes are two distinct categories of substances with unique properties and roles within physiological systems.Electrolytes are Substances that dissociate into ions when Dissolved in solution and play key roles in various Physiological Processes, from fluid balance and nerve transmission, muscle contractions and acid-base balance, as well as many others vital functions within the body.Electrolyte solutions like oral rehydration solutions or intravenous electrolyte solutions are commonly used medical settings to address electrolyte imbalances and support overall health.