The methods of turidimetry as well as colorimetry play a crucial role in determining the amount of substances by how they interact with light. Both techniques make utilization of light sources, the two are distinct in their fundamentals, methods, and instruments.
The main distinctions between turbidimetry, which measures light scattering, and colorimetry, which studies light absorption, which sheds light on their strengths, weaknesses, and applications.
Turbidimetry
Turbidimetry is a measurement technique designed to quantify the degree of turbidity or cloudiness present in a sample. It works on the principle that suspended particles in liquid scatter light, decreasing transmitted light intensity.
Turbidity can then be determined by quantifying this reduction caused by scattered particles present. Turbidimetry is commonly employed for environmental monitoring, water quality assessment, and pharmaceutical analysis applications as a measure of cloudiness or cloudiness present.
Colorimetry
Colorimetry is a scientific technique for measuring and quantifying the color properties of substances. It works on the principle that substances absorb or transmit specific wavelengths of light which result in their perceived hue.
Colorimetry involves using colorimeters or spectrophotometers to assess how a sample absorbs or transmits light at various wavelengths. Colorimetry measures the intensity of light absorbed or transmitted to determine the concentration or presence of colored compounds within a sample, making it useful in many fields including chemistry, biochemistry, food science, and medical diagnostics for applications like chemical analysis, quality control, and color matching. This technique has become widespread use throughout history for this purpose.
Comparison Table of Turbidimetry and Colorimetry
Below is a comparison table highlighting the key differences between turbidimetry and colorimetry:
| Criteria | Turbidimetry | Colorimetry |
|---|---|---|
| Measurement Principle | Measures the decrease in light intensity caused by the scattering effect of suspended particles in a sample. | Measures the absorption or transmission of specific wavelengths of light by colored compounds in a sample. |
| Parameter Analyzed | Turbidity (degree of cloudiness) | Color intensity or concentration |
| Instrumentation | The light source, photodetectors, cell or cuvette | The light source, filters, photodetectors |
| Sample Requirements | Homogeneous sample | Colored compounds in the sample |
| Applications | Particle concentration analysis, microbial growth assessment, and quality control in pharmaceuticals and beverages. | Chemical analysis, environmental monitoring, and medical diagnostics. |
| Advantages | Suitable for opaque samples, rapid and simple analysis, and high sensitivity. | Wide range of applications, non-destructive analysis, quantitative and qualitative analysis. |
| Limitations | Requires homogeneous samples, may be affected by interfering substances. | Limited to colored compounds, interference from background color. |
This table provides a concise overview of the main differences between turbidimetry and colorimetry in terms of their measurement principles, instrumentation, sample requirements, applications, and advantages/limitations.
It serves as a quick reference to understand the contrasting features of these two analytical techniques.
Advantages and Disadvantages of Turbidimetry
Advantages of Turbidimetry:
Sensitivity: Turbidimetry is highly sensitive and can detect even minute variations in turbidity levels in samples, making it the perfect tool for analyzing samples with low particulate matter or subtle shifts in turbidity levels.
Turbidimetry Has Wide Applications: Turbidimetry can be applied to an extensive array of samples, including liquids, suspensions, and emulsions. It is widely utilized for environmental monitoring, water quality analysis, pharmaceutical research studies, and industrial processes.
Rapid Analysis: Turbidimetry’s fast results make for fast analysis and decision-making, making it ideal for time-sensitive applications or when dealing with large sample sets that must be processed quickly. This makes turbidimetry particularly helpful in these instances.
Turbidimetry Is Non-Destructive: Turbidimetry is a non-destructive technique that doesn’t alter the sample being tested, making further analysis or testing on it possible if necessary. Turbidimetry instruments tend to be cost-effective compared to other analytical techniques, with simple operations and maintenance costs, ultimately saving money over time.
Turbidimetry’s Disadvantages:
Interference: Turbidimetry can be affected by various factors, including colored substances that scatter light differently than the particles being measured – these substances may lead to interference and inaccurate measurements, thus impacting turbidity measurements and leading to inaccurate readings.
Particle Size Distribution: Turbidimetry does not provide insight into particle size distribution within a sample; instead it only measures overall turbidity – making its use in applications where particle analysis is important more limited.
Turbidimetry’s limited specificity: Turbidimetry measures overall particle turbidity but doesn’t give us information on their composition and identity – this may require additional methods or analyses in order to fully characterize your sample.
Calibration Requirements: Turbidimeters require regular calibration in order to provide accurate measurements. Calibration involves using standard solutions with known turbidity values as part of the analysis process and requires maintaining calibration standards properly.
Preparation: Preparing samples may require specific techniques such as filtration or dilution to achieve accurate turbidity measurements, thus increasing complexity and time needed for analysis.
Advantages and Disadvantages of Colorimetry
Advantages of Colorimetry:
Quantitative Analysis: Colorimetry allows for quantitative analysis of colored substances within samples, providing numerical data that can easily be understood and compared across samples.
Colorimetry’s high specificity: Colorimetry allows researchers to efficiently and precisely assess any substance with color-absorbing properties that are of interest, whether that’s organic or inorganic substances, drugs, pollutants, or biochemical markers.
Colorimetry Applications: Colorimetry has numerous applications in numerous fields, such as chemistry, biochemistry, clinical diagnostics, environmental monitoring, and food analysis. As it can adapt to diverse sample types and matrices it offers great versatility in application.
Simple Instrumentation: Colorimetric analysis typically relies on simple and compact instruments, such as spectrophotometers or colorimeters, which are readily available, cost-effective, and user-friendly, making colorimetry accessible to many laboratories. Colorimetry offers rapid analysis, making for quick assessments and decision-making processes. It is particularly helpful when large samples need to be evaluated or real-time monitoring is required.
Disadvantages of Colorimetry:
Colorimetry Is Limited to Colored Substances: Colorimetry requires colored substances in samples for effective analysis; as it cannot apply to colorless compounds, making this form of analysis unsuitable for certain samples.
Interference from Background Absorbance: Colorimetry measurements may be affected by other absorbent species or background absorbance in a sample, leading to inaccurate readings if appropriate corrections aren’t made. This can cause inaccuracies or false readings if proper corrections aren’t applied.
Colorimetry lacks selectivity: Colorimetry only measures overall absorbance or color intensity without providing information about individual compounds present in a sample, making further separation or identification techniques necessary to isolate specific components for analysis.
Calibration Requirements: Similar to turbidimetry instruments, colorimetry instruments require periodic calibration using standard solutions of known concentration. Calibration helps ensure accurate measurements while proper maintenance of calibration standards is also essential.
Sensitivity to Experimental Conditions: Colorimetry measurements may be affected by various experimental conditions, including temperature, pH levels, and sample matrix. Standardizing and controlling these conditions is crucial in order to guarantee reproducible and accurate results.
Environmental monitoring and water quality analysis
Environmental monitoring and water quality analysis are vital in assessing ecosystem health and ensuring clean and safe water resources are readily available to people. Turbidimetry and colorimetry play key roles here; below are some applications and considerations specific to environmental monitoring and analysis.
Applications of Turbidimetry:
Suspended Solids and Turbidity Measurement: Turbidimetry is commonly used to measure suspended solids and turbidity levels in water samples. Increased levels can indicate pollutants, sedimentation, or organic material in the environment that adversely impact both aquatic ecosystems as well as water quality.
Sediment Transport and Erosion Studies: Turbidimetry measures the concentration of suspended sediments in rivers, lakes, and coastal environments to accurately gauge erosion patterns, sediment transport rates, and sedimentation rates, which in turn impact water quality and ecosystem health. This information provides essential data that helps researchers analyze erosion processes as well as the habitat effects of their research projects.
Monitoring Effluent Discharge: Turbidimetry is used to track turbidity levels in effluent discharge from industrial plants or wastewater treatment facilities, with compliance to regulatory standards and efficiency evaluation being among its main goals.
Applications of Colorimetry:
Nutrient Analysis: Colorimetry is widely utilized for measuring nutrients such as nitrates, phosphates, and ammonia in water samples. Elevated levels of these substances may contribute to eutrophication, algal blooms, and degraded water quality, making nutrient analysis an integral component of environmental monitoring.
Metal Ion Detection: Colorimetry can be used to identify and quantify metal ions such as copper, lead, and mercury in water samples. Elevated concentrations may arise due to industrial discharges or natural sources and be detrimental to aquatic life and human health.
pH and Chemical Parameter Analysis: Colorimetric methods are utilized for measuring parameters like pH, alkalinity, hardness and chemical oxygen demand (COD), providing insights into the chemical makeup and characteristics of water bodies.
Considerations:
Method Sensitivity and Range: Both turbidimetry and colorimetry methods must have sufficient sensitivity and dynamic range in order to detect and measure target parameters within their expected concentration ranges in environmental samples.
Collection and Preservation Methods: Appropriate collection techniques and preservation methods are key to obtaining reliable results. Samples should be collected according to established protocols, then stored securely so as to prevent changes in turbidity or chemical composition during storage.
Calibration and Quality Control: Regular calibration of turbidimeters and colorimeters against certified reference standards is vital to maintain accuracy. Quality control measures, including analysis of known reference samples, should also be implemented to validate instrument performance and results.
Interferences and Matrix Effects: Environmental samples may contain various substances that interfere with turbidimetric or colorimetric measurements, requiring special consideration when taking these measurements. It’s crucial to recognize potential interferences and apply appropriate corrections or pre-treatment methods in order to achieve reliable results.
Compliance: Environmental monitoring and water quality analysis must often comply with specific regulatory standards and guidelines, and methods used must be validated by relevant governing bodies to ensure compatibility of results and compliance.
Environmental scientists and water quality analysts use both turbidimetry and colorimetry techniques to collect comprehensive data on suspended solids, turbidity, nutrients, metals, and other key parameters that impact aquatic ecosystems and public health. Such analyses help in evaluating human activities’ impacts, monitoring trends in water quality conditions, and taking measures necessary for their preservation.
Measurement of color intensity using colorimetry
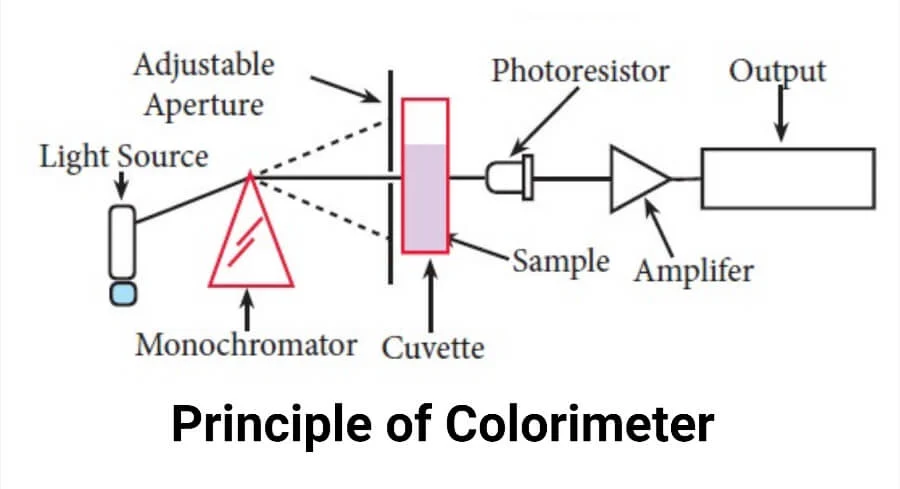
Colorimetry is a technique for measuring the intensity of color present in samples, using light’s interaction with their colored contents as the basis for measurement.
The process typically includes these steps:
Colorimetry involves selecting a wavelength of light that corresponds with the maximum absorption capacity of colored substances, usually determined through prior experience or experimentation.
Illumination: To illuminate a sample with light of the desired wavelength, either using an LED source or by isolating specific frequencies with filters.
Absorption of Light: When colored substances are added to samples, they absorb some of the incident light at certain wavelengths, with this amount dependent on how concentrated the colored substances are present in them.
Measurement of Transmitted or Reflected Light: Light that passes through a sample can be measured using an instrument, such as a photodiode or spectrophotometer, that measures its intensity as it has passed through it. This helps ensure accurate data.
Comparison to Reference Standard: Once measured intensity has been determined, it can be compared against a reference standard such as a blank sample or standard with known concentration or color intensity levels.
Calculation of Color Intensity: To accurately estimate the concentration of colored substances present in samples, their color intensity or intensity must be calculated based on measured light intensity measurements and an established calibration curve that links those intensities with known concentrations of the colored substance.
Data Analysis and Interpretation: Once color intensity values have been calculated, they can be further examined and interpreted according to your application or purpose. This could involve comparing them against regulatory limits, evaluating trends, or assessing sample quality or composition.
Colorimetry assumes a linear relationship between the concentration or color intensity of substance and measured light intensity, however, this linearity may become limited under certain concentration ranges requiring diluting or further analysis techniques for accurate quantification.
Furthermore, proper calibration with standard solutions of known concentration is crucial to ensure reliable measurements.
Factors affecting colorimetry measurements
Colorimetry measurements may be affected by numerous external influences that could impede their accuracy and reliability, making the analysis harder than needed. It is therefore essential to keep all relevant factors in mind during the colorimetric examination.
Here are a few key ones that could influence colorimetric results:
Variations in Light Source Properties: Colorimetry measurements can be affected by variations in light sources’ characteristics like intensity, spectral distribution and stability. Any shifts or inconsistencies between measurements taken using various light sources could introduce inconsistencies that lead to inaccurate results; regular calibration and maintenance of all light sources is crucial to ensure consistent and reliable measurements.
Sample Presentation and Geometry: The way a sample is presented and its geometry can greatly impact colorimetry results. Factors like sample thickness, orientation and reflective surfaces can all have an effect on how much light transmits through or reflects back, leading to variations in measurement results and creating differences in hue intensity measurement results.
Therefore, standardizing these elements of measurement geometry for accurate and reproducible results is imperative for accurate and reproducible color intensity measurements.
Interference from Background or Matrix: Other substances or the matrix itself can obstruct colorimetric measurements, with substances absorbing light at similar wavelengths to your target color often masking or altering its intensity, while matrix effects like turbidity or scattering could impede transmission or reflection, impacting colorimetry measurements. Proper sample preparation methods like filtration or correction could be necessary to minimize interference and achieve accurate results.
Instrument Calibration: Proper calibration is crucial to accurate colorimetry measurements. Instruments used for colorimetric analysis should be calibrated using reference standards with known color intensities or concentrations to ensure linear and consistent response over the desired measurement range. Routine checks and recalibration when necessary can ensure measurement accuracy.
Subjectivity in Colorimetric Measurement: Human interpretation can play an integral part in some colorimetric measurements. Individual perception and subjectivity differences can lead to inconsistencies when it comes to assessing color intensity assessments. Training observers, using color standards for comparison purposes and creating standard protocols are ways of helping reduce observer bias and improving reproducibility of results.
Environment Factors: Environmental conditions such as ambient light, temperature, and humidity can have a direct impact on colorimetry measurements. Shifting lighting conditions or variations in temperature or humidity levels may alter perceived color perception or instrument performance; controlling and monitoring these environmental elements can help minimize their influence on colorimetry measurements.
Sample Stability: Colorimetry measurements depend heavily on the stability of colored substances within samples. Over time, some substances may degrade or undergo chemical reactions which lead to changes in hue. Proper sample storage and handling as well as minimization of light exposure or other reactive agents is key in order to keep samples stable for colorimetric analysis.
By understanding and controlling these factors, colorimetry measurements can be conducted more precisely and reliably. Standardizing procedures, calibrating equipment appropriately, sample preparation as necessary, and eliminating potential sources of interference are all vital steps toward producing consistent and meaningful color intensity results.
Analyzing chemical concentrations in solutions
Analyzing chemical concentrations in solutions is a cornerstone of analytical chemistry. This involves determining the amount or concentration of specific chemical species present in solutions using various analytical techniques like colorimetry.
Here are some methods and considerations for conducting such an analysis:
Titration is an efficient quantitative analysis technique. It involves adding a known-concentration reagent (titrant) to a solution containing an analyte until its reaction with it has taken place and at that point stops.
Titration endpoints may be determined visually through color change or using indicators, while the volume of titrant needed to reach the endpoint allows the calculation of the concentration of the analyte in its original solution.
Spectrophotometry: Spectrophotometry measures the absorption or transmission of light by chemical species present in a solution. Analytes’ concentration can be determined by measuring intensity before and after passing through their solution; calibrated curves linking analyte concentration with measured absorbance/transmittance are created from standard solutions of known concentration, then used to calculate its presence in unknown solutions.
Chromatography: Chromatographic techniques such as high-performance liquid chromatography (HPLC) or gas chromatography (GC) are frequently employed for analyzing chemical concentrations in complex mixtures.
They work by isolating analytes through interactions between them and stationary phases and mobile phases, and suitable detectors like UV-Vis detectors for HPLC or flame ionization detectors for GC. Concentrations are then determined by comparing detector responses against standard solutions.
Electrochemical Methods: Electrochemical techniques such as potentiometry, voltammetry, and amperometry are utilized for analyzing chemical concentrations in solution.
These methods involve measuring electrical properties or current generated by the analyte under specific electrochemical conditions; its concentration can then be determined by comparing measured responses with calibration curves or standard solutions.
Calibration and Standard Solutions: Accurate analysis of chemical concentrations in solutions requires calibration using standard solutions of known concentration.
Calibration curves or standard curves are generated by measuring the response of analytical techniques to a series of standard solutions in terms of concentration range, providing a link between measured response and analyte concentration allowing accurate determination of unknown sample concentrations.
Sample Preparation and Handling: Accurate chemical concentration analysis requires thorough sample preparation and handling procedures, such as filtering, diluting, extracting, or other means. Filtration may be employed to remove interferences or concentrate analyte as necessary or ensure compatibility with chosen analytical methods; special care must be taken not to let samples become contaminated, evaporate or evaporate during storage and preparation processes.
Quality Control and Validation: In order to ensure the reliability and accuracy of results, quality control measures such as the analysis of control samples and validation procedures should be used to safeguard their reliability and accuracy. These should include verifying the accuracy, precision, selectivity, and linearity of analytical methods as well as monitoring instrument performance periodically.
Analyzing chemical concentrations in solutions requires selecting an analytical technique suited to their nature, concentration range, matrix of solution, and desired accuracy and precision.
Understanding its principles, limitations, and calibration procedures as well as proper sample preparation is crucial in order to produce meaningful concentration measurements that provide reliable measurements.
Conclusion
Analytical chemistry relies heavily on measuring chemical concentrations in solutions. Accurate determination of an amount or concentration of a chemical species provides essential data across various scientific disciplines and applications. Techniques such as titration, spectrophotometry, chromatography, and electrochemical methods are frequently employed for this purpose.
As part of the analysis process, various factors must be considered in order to produce accurate and reproducible concentration measurements. This includes selecting an analytical technique, calibrating with standard solutions, sample preparation and handling processes as well as environmental conditions control as well as validation procedures – these all contribute to accuracy and reproducibility in concentration measurement processes.