Introduction of Gaseous and Sedimentary Biogeochemical Cycles
Biogeochemical cycles are fundamental processes that facilitate the movement and transformation of elements and compounds throughout Earth’s ecosystems, contributing to the supply of essential elements needed by living organisms as well as maintaining equilibrium in their biosphere. Two main forms of biogeochemical cycles include gaseous and sedimentary cycles.
Discovering the nuances and processes underlying gaseous and sedimentary cycles allows us to gain greater insights into their distinctive roles in ecosystem functioning, nutrient availability, and human activities on these cycles – crucial components for effective environmental management and the creation of sustainable practices. So let’s dive in deep and discover their fascinating differences!
Definition of biogeochemical cycles
Biogeochemical cycles are natural processes involving the exchange and transformation of elements and compounds between living organisms (bio), the Earth’s surface (geo), and the atmosphere (chemical). They play an essential role in maintaining ecosystem health as well as maintaining the balance between elements in the biosphere.
Biogeochemical cycles involve elements like carbon, nitrogen, phosphorus, oxygen, and sulfur that cycle and recycle through various reservoirs like the atmosphere, oceans, land, and living organisms. They involve both living (plants) and nonliving (rocks, etc) as well as nonliving elements including rocks soil air water, etc.
Biogeochemical cycles are driven by an array of biological, geological, chemical, and physical processes that interact. Examples of such biogeochemical cycles are carbon, nitrogen, phosphorus, oxygen, sulfur, and water cycles – each cycle has its own set of processes, pathways, and factors that determine its progress through Earth systems.
Understanding biogeochemical cycles is central to comprehending how ecosystems function, the availability of essential elements for life and their effects on the environment, as well as understanding human activities on it. By studying these cycles, scientists can gain valuable insight into nutrient cycling dynamics, climate change impacts, pollution issues, sustainability of natural systems, and more.
Definition of Gaseous Biogeochemical Cycle
Gaseous biogeochemical cycles refer to the movement and transformation of elements within Earth’s ecosystems in the form of gases, including carbon, nitrogen, oxygen, and sulfur among others from the atmosphere, living organisms, and the Earth’s surface.
These cycles are driven by various biological, chemical, and physical processes and play an integral part in regulating essential elements, nutrient cycling, energy flow, and climate patterns.
Gaseous Biogeochemical cycles such as the carbon cycle, Nitrogen cycle, oxygen cycle, and sulfur cycle play an Integral part in Maintaining ecosystem Balance on Earth and have profound implications for Environmental Management and Sustainability.
Comparison Table of Gaseous and Sedimentary Biogeochemical Cycles
Sure! Here’s a comparison table highlighting the key differences between gaseous and sedimentary biogeochemical cycles:
| Aspect | Gaseous Biogeochemical Cycles | Sedimentary Biogeochemical Cycles |
|---|---|---|
| Definition | Cycles involving the exchange of gases | Cycles involving the movement of elements in sediments |
| Elements | Carbon, nitrogen, oxygen, sulfur, etc. | Phosphorus, calcium, magnesium, etc. |
| Form of Elements | Gases (CO2, N2, O2, etc.) | Solid particles and dissolved forms |
| Dominant Processes | Photosynthesis, respiration, combustion, etc. | Weathering, erosion, sedimentation, burial, etc. |
| Time Scales | Relatively faster processes | Relatively slower processes |
| Reservoirs | Atmosphere, living organisms, Earth’s surface | Rocks, soil, sediment, water bodies, etc. |
| Environmental Factors | Temperature, atmospheric composition, etc. | Climate, erosion rates, geological activities, etc. |
| Impact on Ecosystems | Influence nutrient availability and climate | Influence soil fertility and geological processes |
| Human Influence and Perturbations | Burning fossil fuels, deforestation, pollution | Mining, erosion, land-use changes, pollution |
Please note that this is a simplified comparison table, and each biogeochemical cycle has its own specific processes and complexities.
Importance of biogeochemical cycles in Earth’s ecosystems
Biogeochemical cycles play an essential part in Earth’s ecosystems and their significance should not be understated.
Here are a few reasons why biogeochemical cycles should not be neglected:
Nutrient Cycling: Biogeochemical cycles serve to ensure an adequate supply of essential nutrients such as carbon, nitrogen, phosphorus, and others that living organisms require for growth, metabolism, and reproduction. By cycling them through various reservoirs of the environment, biogeochemical cycles ensure continuous supply to support life.
Energy Flow: Biogeochemical cycles are intrinsically tied to energy flow in ecosystems. Processes like photosynthesis and respiration play an integral part in these cycles, turning sunlight and organic material into usable energy for organisms. The energy then travels up the food chains or food webs as part of food chains and webs supporting life at various trophic levels.
Climate Regulation: Certain biogeochemical cycles, like the carbon cycle, play an instrumental role in global climate patterns. The exchange of greenhouse gases between the atmosphere, plants, and oceans helps regulate Earth’s temperature; understanding these cycles is therefore integral for understanding climate change dynamics as well as developing strategies to mitigate and adapt accordingly.
Soil Fertility: Biogeochemical cycles, especially sedimentary cycles, play an integral part in maintaining soil fertility and health. Elements like phosphorus, potassium, and calcium cycle through weathered rocks into uptake by plants before their decomposition breaks down organic matter into nutrients that support plant growth and ecosystem productivity. These processes enrich the soil with vitality while supporting ecosystem productivity.
Ecosystem Resilience: Biogeochemical cycles play an essential role in maintaining the resilience and stability of ecosystems through feedback mechanisms such as nitrogen cycles. By controlling its availability, these cycles help maintain equilibrium among different parts of an ecosystem while preventing imbalances that could cause ecological disturbances.
Carbon Sequestration: Biogeochemical cycles such as the carbon cycle play an essential part in carbon sequestration – the process by which CO2 is extracted from the atmosphere and stored in natural areas such as forests, soils, and oceans to mitigate greenhouse gases that contribute to climate change and reduce its impacts. This helps mitigate their accumulation.
Environmental Management: Knowing and controlling biogeochemical cycles is central to sustainable environmental management. By studying them, scientists can identify impacts such as deforestation, pollution, and land-use changes from human activities, then develop strategies to minimize adverse consequences while simultaneously supporting ecosystem health.
Biogeochemical cycles are fundamental processes that regulate the availability of nutrients, energy flow, climate patterns, and soil fertility within Earth’s ecosystems. Their intricate interactions and dynamics play an essential role in their functioning and resilience while providing invaluable insight for addressing environmental challenges and promoting sustainability.
Key elements involved (carbon, nitrogen, oxygen, sulfur)
Gaseous biogeochemical cycles involve several key elements, including carbon, nitrogen, oxygen, and sulfur. Here is an overview of each element and its significance within these cycles:
Carbon (C):
Carbon is an integral part of gaseous biogeochemical cycles, specifically the carbon cycle. Carbon dioxide (CO2) exists Primarily as Greenhouse gas in Earth’s Atmosphere and thus affects its climate Significantly.
Carbon can be Recycled through Processes such as Photosynthesis, respiration, Combustion, and Decomposition. Photosynthetic organisms like plants and algae use photosynthesis to take in CO2 from the atmosphere and convert it to organic compounds for use as food sources.
Respiration by plants, animals, and microorganisms releases CO2 back into the atmosphere, while combustion of fossil fuels and biomass also releases carbon dioxide back into our environment.
Nitrogen (N):
Nitrogen is essential to living organisms as an essential building block of proteins and nucleic acids. The nitrogen cycle involves the conversion of nitrogen between various forms that organisms can use.
nitrogen exists abundantly as nitrogen gas (N2), but cannot directly be utilized by most organisms. Nitrogen fixation involves certain bacteria converting atmospheric nitrogen into forms like ammonia (NH3) or nitrates (NO3-) that plants can utilize directly.
Nitrification involves the conversion of ammonium (NH4+) into nitrate (NO3-), which plants utilize as an essential source of nutrition. Denitrification occurs through denitrifying bacteria which then convert it back to atmospheric nitrogen through their activity. Oxygen (O): Oxygen plays an essential role in plant life as an environmental indicator and source.
Oxygen plays an essential role in biological processes, such as respiration and photosynthesis. Through photosynthesis, plants and some photosynthetic organisms produce oxygen as a byproduct while turning CO2 into organic compounds for growth.
Oxygen is consumed through respiration by plants, animals, and microorganisms to convert organic matter to energy production while producing CO2. Atmospheric and marine oxygen exchange occurs via diffusion or photosynthesis by marine organisms. Sulfur (S): Sulfur’s role is essential to ecosystem health as its presence allows organisms to function optimally.
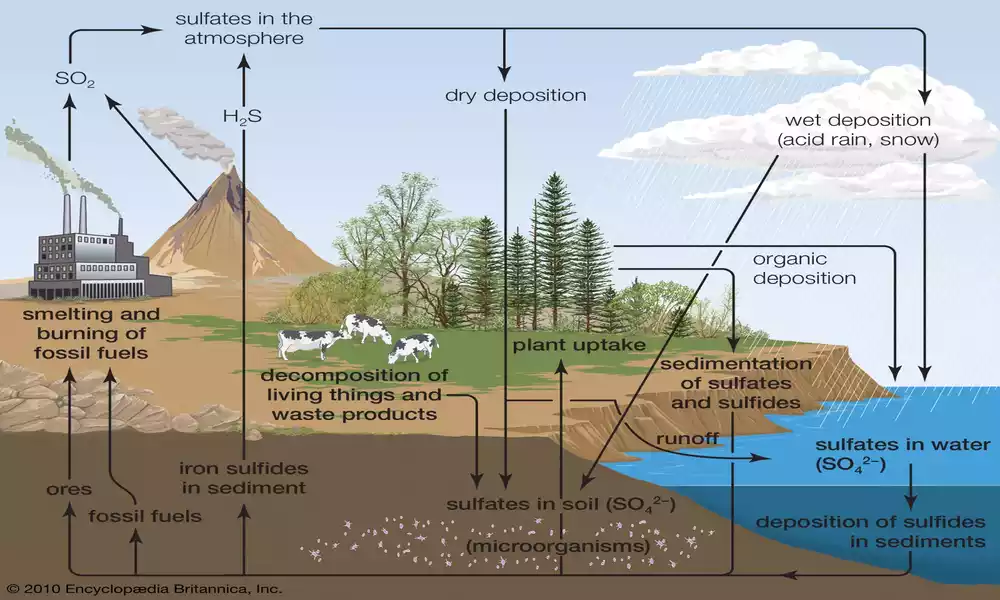
Sulfur cycles through its own system called “The Sulfur Cycle”, which involves the movement and transformation of sulfur-containing compounds. Sulfur exists in different forms such as sulfate (SO42-) and hydrogen sulfide (H2S). When exposed to weather or volcanic emissions, some forms of sulfur enter our environment through weathering of rocks or volcanic emissions.
Biochemical processes, such as bacterial sulfate reduction, can convert sulfate into hydrogen sulfide through biological pathways such as bacterial sulfate reduction. Sulfur compounds enter the atmosphere through volcano eruptions or industrial activities that release them.
Sulfur compounds will eventually return to Earth through precipitation or dry deposition, playing an essential role in life and biogeochemical cycles, including climate, nutrition availability, and ecosystem functioning.
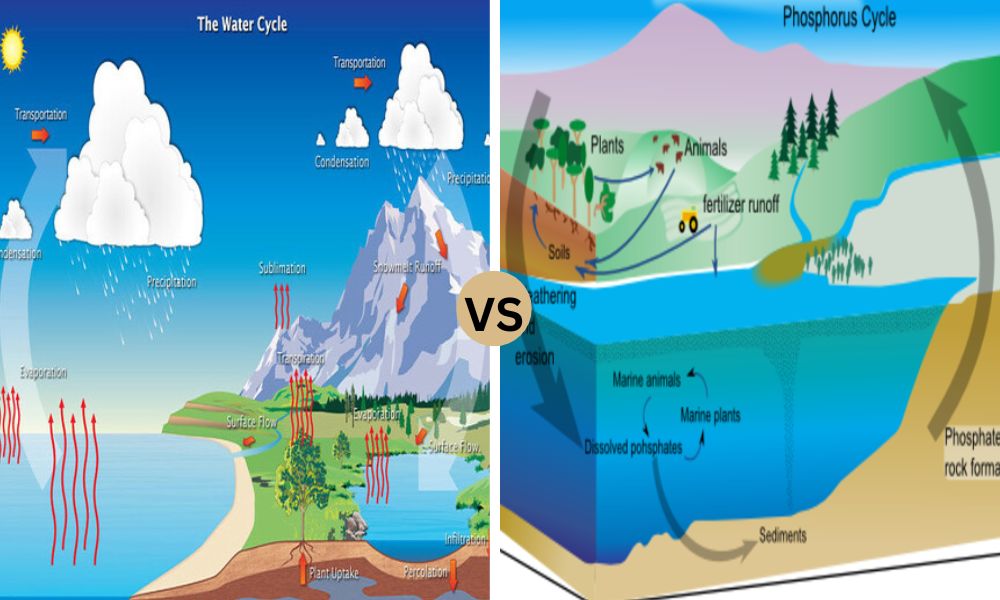
Sedimentary Biogeochemical Cycle
The biogeochemical cycle of sediments refers to the transformation and movement of elements mostly through erosion, deposition, and geological processes that involve sediments. In contrast to gaseous cycles, which require the exchange of elemental gases, the sedimentary cycle focuses on the cycle of elements that are present in both dissolved and solid forms in the Earth’s ecosystems.
A few of the key elements that play a role in the biogeochemical cycles of sediments are calcium, phosphorus, and magnesium. This article will provide an overview of the importance of these elements in the sedimentary cycle:
- Phosphorus (P):
- Phosphorus is an essential element in the diet of living organisms. It plays a crucial role in processes such as DNA transfer, energy transfer, and the synthesis of RNA.
- The phosphorus cycle is the movement of phosphorus across different reservoirs like soil, rocks, and water bodies. living organisms.
- The process of weathering rocks releases phosphorus into the surface and into the water making it accessible to plants for use.
- Plants absorb phosphorus from soil, and it travels across the food chain, as animals eat plants or other animals.
- In time, phosphorus is transferred to sediments via burial and sedimentation processes.
- The uplift of the earth’s crust and its erosion expose phosphorus-rich sediments, restarting the cycle.
- Calcium (Ca):
- Calcium is a vital element in all living organisms. It plays a role in processes like shell and bone development, the activity of enzymes as well as cell-mediated signaling.
- The calcium cycle is the process of cycling calcium through erosion, and weathering along with biological and chemical processes.
- The process of weathering and erosion of rocks releases calcium into the soil and water bodies.
- Calcium is absorbed by plants and then incorporated in their tissue.
- Animals acquire calcium by eating plants and other animals.
- Shells and organic matter rich in calcium can accumulate and cause sedimentary deposits over time.
- Magnesium (Mg):
- Magnesium is an important component of a range of biological processes, like photosynthesis, and enzyme activation in muscle activity.
- The magnesium cycle is the process of cycling magnesium through erosion, weathering, and dissolving forms within the water bodies.
- The process of weathering rocks releases magnesium into the soil and into the water.
- Magnesium is taken up by plants and then incorporated into their tissue.
- Reprecipitation and dissolution processes are key to the cycle of magnesium in sedimentary and aquatic bodies.
- Biochemical processes, like the breakdown of organic material, may allow magnesium to be released back into groundwater and soil.
These elements, as well as other elements in the sedimentary cycle, are essential to fertile soils and the formation of sedimentary rocks, as well as how ecosystems function on Earth.
The biogeochemical processes in sedimentary cycles run for longer durations than gaseous cycles and are influenced by various factors, including erosion rates, climate, and geological processes.
When we understand this cycle, we will be able to better understand the fertility of soils as well as geological processes and the long-term cycle of the earth’s ecosystems.
Key elements involved (phosphorus, calcium, magnesium)
At the core of sediment biogeochemistry are calcium, phosphorus, and magnesium: key elements which play key roles in its biogeochemical cycle.
We will review each one and their significance within sedimentary formation:
Phosphorus (P): Phosphorus is an essential food element for living organisms, playing an essential role in various processes like DNA replication and energy transfer as well as cell signaling. Phosphorus often forms part of sediment cycles associated with minerals, rocks, and organic material.
Weathered rocks release phosphorus into the ground and water bodies, making it accessible for plants. When plants absorb it through their roots into their tissues, this then travels down their food chains as animals consume both plants and other animals that ingest phosphorus from soil sources.
Over time, phosphorus could reenter sediments through processes like burial and sedimentation. When the earth’s surface conditions change due to uplift or erosion, the newly exposed phosphorus-rich sediments resume their cycle.
Calcium (Ca):
Calcium plays a pivotal role in many biological processes, from bone formation and muscle function to enzyme activity and sediment cycle processes. Within sediment systems, calcium-rich minerals, rocks, and shells often comprise sources of this mineral element.
Calcium-rich rocks erode into the ground and water bodies over time, discharging calcium into both. When plants absorb it from soil they incorporate it directly into their tissues; animals get their calcium through eating plants and animals alike.
As shells and organic material rich in calcium accumulate over time, contributing to sedimentary deposits, magnesium is also an essential element that plays a vital role in many biological processes, including photosynthesis, enzyme activation, and muscle functions.
Magnesium plays a significant role in the cycle of sediment, predominantly associated with minerals, rocks, and the forms found in water bodies. Weathering of magnesium-rich rocks releases this element to soil and water bodies for plant uptake; plants then can use these sources of magnesium to incorporate it into their tissue systems.
Reprecipitation and dissolution processes aid the cycling of magnesium within sediments and water bodies, while biochemical reactions like breaking down organic matter release magnesium back into earth and water bodies.
Calcium, phosphorus, and magnesium – elements involved in sedimentary cycles – play an integral part in soil fertility, the formation of sedimentary rocks, and ecological functioning. Understanding their circular relationship allows us to better comprehend available nutrients, geological processes, and long-term changes within Earth’s ecosystems.
Environmental factors influencing each cycle
Environmental factors play a vital role in shaping both gaseous and sedimentary biogeochemical cycles, impacting their dynamics and rates of development. Below are some key environmental influences that may have an effect on each cycle:
Biogeochemical Cycles in Gaseous Form:
Temperature: The rate of biochemical reactions involved in gaseous cycles can be affected by temperature. Warmer temperatures tend to accelerate photosynthesis, respiration, and microbial activity rates and thus affect the exchange and transformation of gases.
Atmospheric Composition: The composition of the atmosphere can play a significant role in gaseous cycles, particularly the concentrations of CO2 and NOx gases such as carbon dioxide (CO2) and nitrogen oxides (NOx). Changes to atmospheric composition due to human activities, such as burning fossil fuels or industrial processes, can have profound ramifications on these cycles.
Climate Patterns: Climate factors such as precipitation, humidity, and wind patterns influence gas distribution as well as access to water and nutrients. Climate change could alter these patterns of precipitation and temperature thus altering photosynthesis, respiration, and other processes in gaseous cycles.
Land Use and Vegetation: Land use changes such as deforestation, urbanization, and agricultural practices can significantly impact gaseous cycles. Vegetation cover plays an essential role in carbon sequestration by modulating exchanges of carbon dioxide and oxygen between ecosystems and the atmosphere.
Sedimentary Biogeochemical Cycles:
Climate and Weathering: Climate has an enormous influence on weathering processes that release elements from rocks and minerals into sedimentary cycles, such as weathering. Temperature and precipitation patterns play a vital role in these weathering cycles, with warmer and wetter environments often increasing weathering rates, leading to quicker erosion rates that release these elements more readily from rocks and minerals. Wetter and warmer environments generally increase weathering rates more significantly – impacting availability and cycling cycles more directly.
Erosion and Sediment Transport: Erosion, sediment transport, and deposition processes influence the movement and distribution of sediment-containing elements. Weather patterns, slope steepness, land-use practices, and rainfall all play a part in changing erosion rates which then results in altered cycles for sedimentary cycling.
Geological Activity: Geological processes like tectonic activity, volcanic eruptions, and uplift can have an enormous effect on sedimentary cycles by exposing new rock layers, discharging elements into the environment, and contributing to sediment formation.
Water Chemistry: The chemical makeup of bodies of water such as rivers, lakes, and oceans influences both the dissolution and precipitation of elements which influence their cycling through sedimentary systems. Elements like pH, salinity, and oxygen levels all impact chemical reactions occurring within aquatic environments.
Environmental factors interact with the living components of ecosystems – plants, microorganisms, and animals – to influence biogeochemical cycles that span from gaseous to sedimentary. Recognizing their influence is key for accurate forecasting and managing the responses of Earth’s ecosystems to environmental changes.
Conclusion
Biogeochemical cycles – both gaseous and sedimentary cycles – play an integral part in maintaining Earth’s ecosystems by controlling the movement and transformation of elements within ecosystems.
Gaseous cycles involve exchanging elements in the form of gases such as carbon, nitrogen, oxygen, and sulfur between the atmosphere, living organisms, and Earth’s surface while sedimentary cycles involve shifting of elements primarily through deposition, erosion, and geological processes involving sediments.
These cycles are Affected by various Environmental factors, including Temperature, atmospheric Composition, climate patterns, land use practices, erosion, sediment transport, and Geological activity. Understanding their effect is vital in order to gain insight into nutrient availability, energy flows, climate regulation, soil fertility, and ecosystem resilience.