Introduction of F Plasmid and R Plasmid
Plasmids are essential genetic elements found in bacteria and some other organisms that exist independently from the chromosomal DNA. They consist of circular or linear DNA molecules capable of replicating independently and being transferred between cells, acting like DNA microchips to provide advantages such as antibiotic resistance, toxin production or accessing certain nutrients.
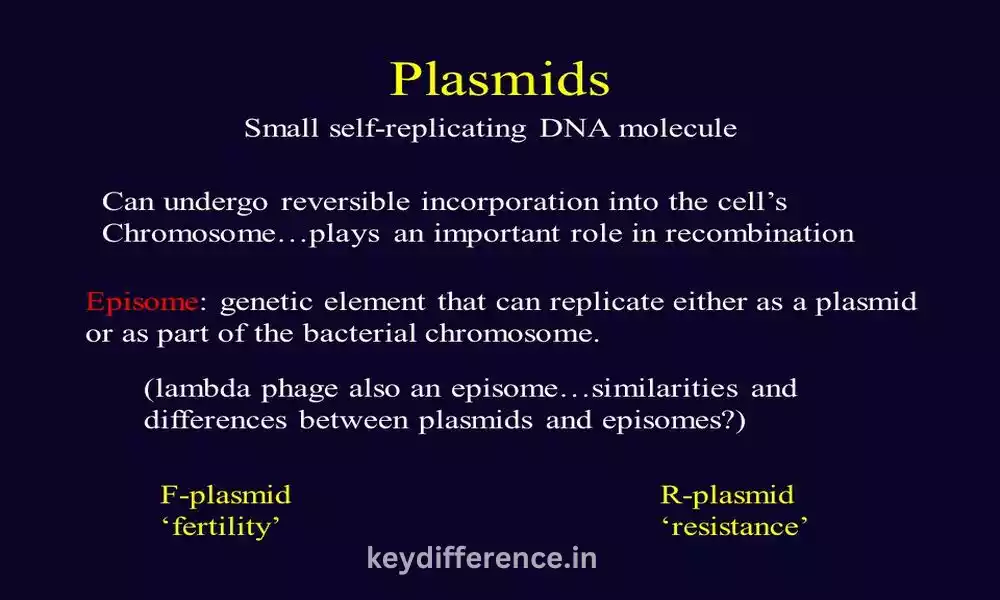
F and R plasmids are two distinct categories of plasmids, known as Fertility Plasmids or F Factors, respectively. F Plasmids play an integral part in bacterial conjugation; on the other hand, R Plasmids or Resistance plasmids or R factors contribute significantly to antibiotic resistance among bacteria.
Understanding the differences between F and R plasmids is essential in understanding genetic transfer mechanisms and antibiotic resistance spread in bacterial populations. This content outline will explore their respective characteristics, functions, and implications as a comprehensive overview of their differences as they pertain to bacterial genetics and public health.
Definition of F Plasmid
F Plasmids (or Fertility Plasmids or F Factors), are an extrachromosomal DNA molecule commonly found in Escherichia coli bacteria, particularly E. coli strains. First discovered during studies on gene transfer between bacteria in the 1950s.
F plasmids are circular, double-stranded DNA molecules that exist independently from bacterial chromosomes. They are relatively small in size – typically 90 to 100kb in length – and carry specific sets of genes that enable transfer from donor (F+) cells to recipient (F-) cells by means of conjugation.
The F plasmid contains genes encoding proteins and factors necessary for conjugation, such as a sex pilus. This thin hair-like appendage connects donor and recipient cells by creating physical contact. Through the formation of a conjugation bridge, F plasmid can then be transferred from donor to recipient cells.
Assuming successful transfer, the recipient cell becomes a new donor able to propagate F plasmids both vertically (from parent to offspring) and horizontally (between unrelated bacteria).
F plasmids carry more than just conjugation genes; they may also carry antibiotic resistance genes or metabolic pathway-related genes, making F plasmids instrumental in spreading genetic traits throughout bacterial populations, helping them evolve and adapt in different environments.
F plasmids are extrachromosomal DNA molecules found primarily in E. coli bacteria that replicate themselves via self-replication and transfer between cells, providing crucial horizontal gene transfer mechanisms and increasing genetic diversity in their host bacteria population.
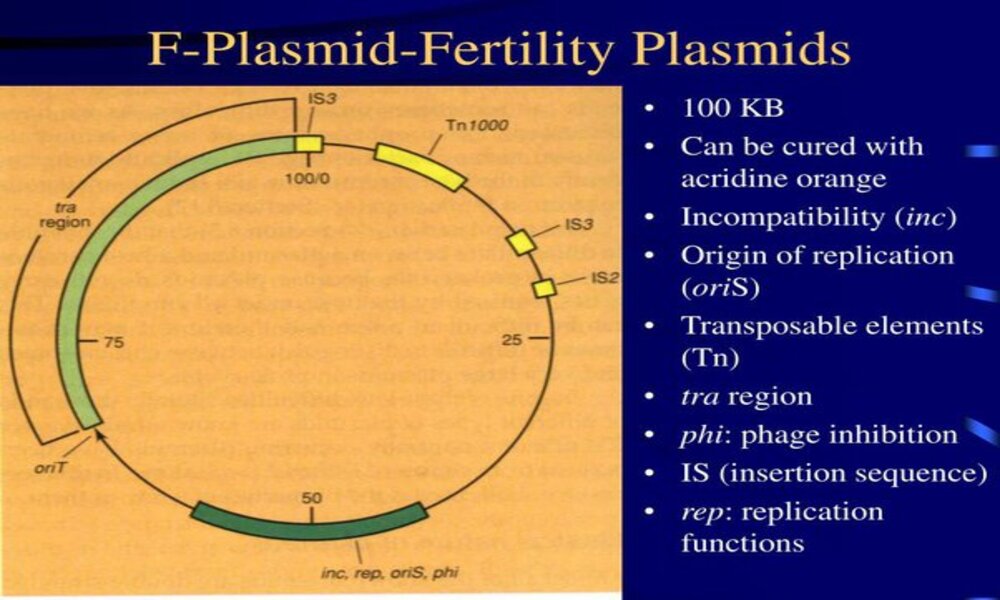
Definition of R Plasmid
R Plasmid (also referred to as Resistance Plasmid or R Factor) is an extrachromosomal DNA molecule commonly found in bacteria. R Plasmids are known for carrying genes that confer antibiotic resistance, making them significant contributors to antimicrobial resistance within populations.
Similar to other plasmids, R plasmids are circular, double-stranded DNA molecules that reside independently from bacterial chromosomes and vary in size from several hundred kb up to several megabase pairs (kb). Furthermore, R plasmids contain specific genes which encode enzymes or proteins which provide resistance against one or more antibiotics.
R plasmids carry resistance genes that confer resistance to antibiotics through various mechanisms. These include the production of enzymes that degrade or modify antibiotics, efflux pumps that expel antibiotics from bacterial cells, or modifications at target sites that render antibiotics ineffective.
R plasmids can rapidly spread antibiotic resistance among bacteria through horizontal gene transfer mechanisms such as conjugation, transformation, and transduction. This means that bacteria carrying R plasmids may transfer them and their resistance genes to other species of bacteria that become infected with them – thus increasing the prevalence of antibiotic-resistant strains.
R plasmids acquired by bacteria can create significant difficulties when treating infections caused by them, as these bacterial strains become resistant to multiple antibiotics, decreasing treatment effectiveness and raising treatment failure risks. This poses a grave public health threat; infections caused by antibiotic-resistant bacteria become harder to treat, leading to greater morbidity and mortality rates than ever before.
R plasmids are extrachromosomal DNA molecules found in bacteria that contain genes conferring resistance to antibiotics. R plasmids play an integral part in spreading antibiotic resistance among populations, contributing to multidrug-resistant strains, as well as creating difficulties for treating infections effectively.
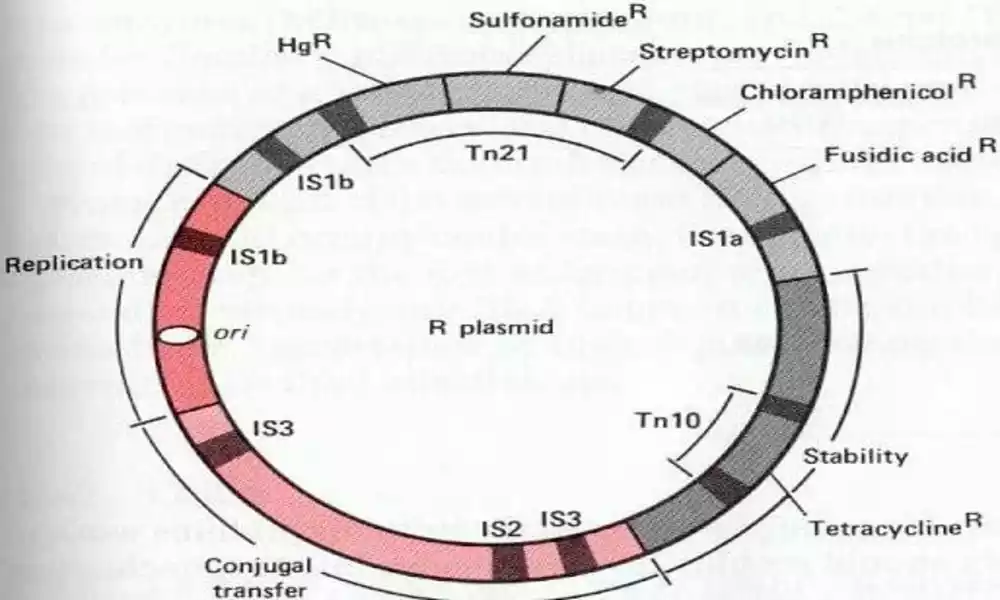
Comparison Table of F Plasmid and R Plasmid
Sure! Here’s a comparison table highlighting the key differences between F plasmids and R plasmids:
| Aspect | F Plasmid | R Plasmid |
|---|---|---|
| Definition | A plasmid involved in bacterial conjugation | A plasmid responsible for antibiotic resistance |
| Transfer Method | Conjugation | Conjugation, transformation, transduction |
| Size | Typically 90-100 kilobase pairs (kb) | Variable sizes, ranging from a few kb to several hundred kb |
| Genetic Elements | Genes involved in the conjugation process | Genes encoding antibiotic resistance |
| Conjugation | Forms a conjugation bridge via a sex pilus | Forms a conjugation bridge via a sex pilus |
| Antibiotic Resistance | May carry antibiotic resistance genes | Carries antibiotic resistance genes |
| Gene Transfer | Vertical and horizontal transfer | Horizontal transfer among bacteria |
| Genetic Diversity | Contributes to genetic diversity in bacterial populations | Contributes to the spread of antibiotic resistance and the emergence of multidrug-resistant bacteria |
| Implications | Facilitates gene transfer and adaptation | Contributes to the spread of antibiotic resistance and challenges in treatment |
| Examples | F plasmid in Escherichia coli (E. coli) | R plasmid in various bacterial species |
Importance of plasmids in bacterial genetics and gene transfer
Plasmids play a critical role in bacterial genetics and gene transfer, contributing to genetic diversity and adaptability within populations.
Their significance can be highlighted from several angles:
Horizontal Gene Transfer: Plasmids allow bacteria to exchange genetic material horizontally through mechanisms like conjugation, transformation, and transduction. By doing so they enable the rapid spread of beneficial traits such as antibiotic resistance, virulence factors, or metabolic capabilities among populations of bacteria.
Antibiotic Resistance: Plasmids often carry genes that provide resistance to antibiotics. If transferred to susceptible bacteria, the resistance genes could potentially result in antibiotic-resistant strains emerging that limit the effectiveness of antibiotics and present challenges when treating infections caused by bacteria. This poses serious healthcare concerns as it undermines the effectiveness of treatment protocols while creating a greater need for new ones to combat infections caused by bacteria.
Genetic Flexibility: Plasmids play an essential role in bacteria’s genetic flexibility and adaptability, carrying many types of genes involved in metabolic pathways, toxin production, symbiosis, and stress response.
Rapidly adapting to changing environments by rapidly acquiring advantageous plasmids with advantageous genes that carry advantageous traits that enhance survival and competitiveness through acquisition of advantageous plasmids carrying advantageous genes plasmids are a great tool.
Evolutionary Adaptation: Plasmids provide a mechanism for the evolution and adaptation of bacteria. By replicating independently from their host chromosome, plasmids can accumulate genetic changes independently allowing bacteria to rapidly acquire adaptive traits for use in specific ecological niches or respond to selective pressure.
Genetic Engineering and Biotechnology: Plasmids play an essential role in genetic engineering and biotechnology. Scientists rely on them as vectors to introduce foreign DNA into bacterial cells for producing valuable proteins, manipulating genetic pathways, studying gene function, or exploring gene properties. Furthermore, plasmids provide efficient tools for gene cloning, expression, and genetic modification.
Plasmids are essential components of bacterial genetics and gene transfer, serving to facilitate the horizontal transfer of genetic material such as antibiotic-resistance genes between populations.
Plasmids contribute to genetic diversity, adaptation, and evolution while also serving as invaluable tools in genetic engineering and biotechnology applications. Understanding their roles within bacteria biology is vital in order to effectively combat antibiotic resistance issues, study gene function more deeply, and capitalize on any potential applications they might present.
Additional genetic elements carried by F plasmid
F plasmids contain more than just conjugation genes; they may contain additional genetic elements that provide various advantages to their host bacteria. Such elements could include:
Antibiotic Resistance Genes: F plasmids may harbor genes that confer resistance to antibiotics. Such genes could encode enzymes or proteins that modify or degrade antibiotics, efflux pumps that expel them from the cell, or even changes to target sites which render antibiotics ineffective; their presence contributes to the further spread of resistance among bacterial populations.
Metabolic Genes: F plasmids may contain metabolic genes that enable bacteria to utilize specific nutrients or undertake metabolic pathways not present in their host bacterium’s chromosomal DNA, giving these F plasmids an advantage in environments with limited resources or when alternative metabolic pathways may be necessary for survival. These metabolic genes provide a selective advantage in environments with scarce nutrition or when survival depends on alternative metabolic pathways.
Toxin Genes: Certain F plasmids carry genes encoding toxins or other virulence factors that contribute to pathogenicity by inducing host cell damage, immune evasion strategies or other processes associated with virulence. These genes may play a part in pathogenesis by increasing host cell destruction or immunity evasion and other processes associated with pathogenicity.
Fertility Inhibition Systems: Some F plasmids contain genetic elements that prevent other plasmids from conjugating into bacteria cells, effectively protecting their own stability within their respective populations by blocking any competitive transfers that might interfere with F plasmid stability. Such fertility inhibition systems work against competing transfers while keeping F plasmid stable within them.
Regulatory Elements: Some plasmids contain regulatory elements, such as promoters or enhancers, that regulate gene expression on the plasmid. This ensures optimal timing and levels of gene expression to maximize the use of genetic information contained within it.
F plasmids contain additional genetic elements which increase genetic diversity and adaptability among bacterial populations, providing selective advantages to their host bacteria as well as aiding the evolution and survival of these microbes in diverse environments.
Transfer of antibiotic resistance through F plasmid
Transfer of antibiotic resistance via F plasmids primarily takes place through conjugation, a mechanism of horizontal gene transfer that involves moving genetic material between bacteria (donor and recipient, including any transferred plasmids).
When an F+ bacterium harboring an antibiotic-resistance F plasmid conjugates with an F- bacterium lacking it, its F plasmid can be transferred from donor to recipient and result in acquired antibiotic-resistance genes conferring immunity against specific antibiotics.
Transferring and disseminating F plasmid and antibiotic resistance gene during conjugation generally follows these steps:
Formation of Conjugation Bridge: When two bacteria meet, each F+ donor bacterium extends a thin hairlike appendage called a “sex pilus.” This hairy appendage physically contacts its counterpart F- recipient bacterium and forms a “conjugation bridge”, linking their cells.
DNA Transfer: When F plasmid replicates, one copy is transferred from an F+ donor bacterium to an F- recipient bacterium via conjugation bridge and integrated into their genome as an F+ cell. Once in, this F+ copy becomes established within the host cell’s genome establishing it as an F+ organism.
Antibiotic Resistance Gene Transfer: If an F plasmid carrying antibiotic resistance genes from an F+ donor bacterium transfers those genes to its recipient bacteria, those genes will also become part of its genome and result in its acquisition of resistance against antibiotics.
Establishment of F+ Bacteria: Once an F plasmid has been introduced into a recipient F- bacteria strain, its status changes to that of an F+ bacterium that can serve as a donor during subsequent conjugation events.
F plasmids carry antibiotic resistance genes that can spread horizontally among bacterial populations, contributing to their spread through transference from resistant bacteria onto susceptible ones and thus further spreading resistance traits across generations of bacteria. As such, F plasmids carrying resistance genes play an essential role in multidrug-resistant strain development and spread, creating new challenges to treatment as well as public health concerns.
Antibiotic resistance and R plasmid
Antibiotic Resistance and R plasmids are Inextricably linked, with R Plasmids carrying genes to confer Resistance against antibiotics and spreading such Resistance among bacteria resulting in the Appearance of multidrug-resistant Strains. Here are some key points about antibiotic resistance and R plasmids:
Acquisition of Resistance Genes: R plasmids contain genes encoding various mechanisms of antibiotic resistance, such as enzymes that modify or degrade antibiotics, efflux pumps that expel antibiotics from bacteria cells, or alterations to their target sites. These resistance genes may be clustered together into resistance gene cassettes for easier administration.
Spread of Resistance: R plasmids can transfer horizontally among bacteria through conjugation, transformation, and transduction processes; this allows resistance genes to spread among related and unrelated bacterial species and contribute to spreading antibiotic resistance across species boundaries.
Multiple Antibiotic Resistance: R plasmids may carry multiple antibiotic resistance genes that confer protection from multiple classes of antibiotics; this phenomenon, known as multidrug resistance, poses a formidable obstacle when treating bacterial infections as it limits their efficacy.
Co-Resistance and Co-selection: R plasmids can carry multiple classes of antibiotic resistance genes as well as non-antibiotic resistance genes, leading to co-resistance phenomena when different resistance genes for different antimicrobial agents coexist on one plasmid and increasing the likelihood of multidrug-resistant strains arising. Furthermore, using one antibiotic can inadvertently select bacteria carrying R plasmids with resistance genes for other antibiotics, furthering resistance spread.
Persistence and Evolution: R plasmids have the capacity to persist even without antibiotic selection pressure, due to stable genes on them which ensure it continues to divide during cell division. Furthermore, over time R plasmids may undergo genetic modifications which allow them to acquire or lose resistance genes leading to novel resistance phenotypes emerging over time.
Public Health Implications: R plasmids and their role in spreading antibiotic resistance present significant challenges in healthcare settings, making multidrug-resistant infections difficult to treat, leading to increased morbidity, mortality, and healthcare costs. Furthermore, R plasmids compromise antibiotic effectiveness limiting treatment options available against various bacterial infections.
Understanding the link between R plasmids and antibiotic resistance is integral for developing strategies to combat its spread and ensure effective treatment of bacterial infections.
Types of antibiotic resistance genes carried by R plasmid
R plasmids may carry antibiotic-resistance genes that confer immunity against specific classes or individual antibiotics. These resistance genes can be divided up based on how they provide resistance.
Here are some commonly found resistance genes on R plasmids:
Beta-lactamase genes: Beta-lactamases are enzymes that hydrolyze beta-lactam rings, rendering certain beta-lactam antibiotics such as penicillins and cephalosporins ineffective. R plasmids often carry genes for beta-lactamases such as TEM, SHV, and CTX-M that confer resistance against such medications.
Efflux pump genes: Efflux pumps are membrane proteins that actively pump antibiotics out of bacterial cells to decrease their intracellular concentration and provide resistance against various classes of antibiotics, including fluoroquinolones and tetracyclines. Plasmids carrying efflux pump-coding genes like AcrAB-TolC conferring resistance confer resistance against several classes of antibiotics including fluoroquinolones and tetracyclines.
Enzyme-modifying genes: Some R plasmids contain genes encoding enzymes that alter antibiotics and render them inactive, such as aminoglycoside-modifying enzymes (e.g. AAC, ANT, and APH). Such modifications chemically modify aminoglycoside antibiotics by decreasing their binding affinity with bacteria targets and thus rendering them nonactive.
Target Site Alteration Genes: R plasmids may carry genes that alter the target site of antibiotics, diminishing their efficacy. For instance, genes encoding modified ribosomal proteins or mutations of DNA gyrase/topoisomerase may confer resistance against macrolides and fluoroquinolone antibiotics respectively.
Mobile Resistance Genes: R plasmids may acquire mobile genetic elements, such as integrons or transposons that carry multiple antibiotic resistance genes. These elements facilitate capture and exchange, leading to the assembly of complex resistance gene cassettes on R plasmids.
Co-resistance genes: R plasmids can contain multiple resistance genes to confer immunity against various classes of antibiotics. Such co-resistance genes may include those that confer resistance against beta-lactams, aminoglycosides, tetracyclines, sulfonamides, and other antibiotics.
Note that R plasmids carry different resistance genes depending on the strain and environment in which they’re found, leading to multidrug-resistant bacteria and creating difficulties when treating infections caused by them.
Spread of antibiotic resistance through R plasmid
Antibiotic resistance spreads via R plasmids via horizontal gene transfer, dispersing resistance genes among bacterial populations.
Here are the main mechanisms by which antibiotic resistance spreads:
Conjugation: Conjugation is the primary means of R plasmid transfer. An R+ donor bacteria form an attachment called the sex pilus with an R- recipient bacterium; through this bridge, a copy of the donor plasmid is transferred over. As a result, its resistance genes become active within both bacteria, creating new R+ bacteria capable of further spreading it further.
Transformation: Under transformation, bacteria acquire extracellular DNA from their surroundings. If that extracellular DNA contains an R plasmid with antibiotic-resistance genes, the recipient bacterium may incorporate or keep it separate as an extrachromosomal element and gain access to them for immune system enhancement. Through this process, antibiotic resistance genes contained within it are acquired.
Transduction: Transduction involves the transfer of genetic material between bacteria via bacteriophages (viral infections of bacteria). When invading one bacterium, the phage can unwittingly package R plasmids along with their associated antibiotic resistance genes for injection into another one when it infiltrates, potentially giving rise to transduction events that lead to antibiotic resistance genes being introduced into that recipient bacterium.
Selection Pressure: Antibiotic use exerts selective pressure on bacterial populations, favoring the survival and proliferation of those carrying R plasmids with antibiotic-resistance genes. Because such resistance confers survival advantages against treatment with specific antibiotics used, resistant strains tend to thrive despite treatment, multiplying and spreading resistance with each multiplication cycle – further contributing to the spread.
Co-Selection: Co-selection occurs when using one antibiotic unknowingly selects for bacteria with R plasmids that contain resistance genes for other antibiotics, for instance, ampicillin may encourage maintenance and spread of said R plasmid, leading to increased resistance against tetracycline as well.
Antibiotic resistance caused by R plasmids is a significant threat to healthcare settings as it limits antibiotic effectiveness, potentially leading to treatment failure and spreading untreatable infections. To curb its spread, efforts include prudent antibiotic usage, infection control measures, and devising novel ways of fighting multidrug-resistant bacteria.
Conclusion
F and R plasmids are two distinct types of plasmids that play essential roles in bacterial genetics and gene transfer. F plasmids aid conjugation between donors and recipients bacteria by carrying genes involved with conjugation as well as additional genetic elements like antibiotic resistance genes. On the other hand, R plasmids confer antibiotic resistance via various mechanisms, making F plasmids essential in maintaining the diversity of bacteria populations.