Introduction of DAPI and Hoechst
Fluorescent stains API and Hoechst are indispensable tools in cell biology and biochemistry. Used to visualize and label DNA, both provide unique properties that make them invaluable in various applications.
While DAPI has a strong binding affinity for AT-rich regions of DNA while Hoechst variants such as Hoechst 33342 offer flexibility under different experimental conditions.
This content explores these widely-used fluorescent stains; discussing their chemical structure, applications, comparative differences, and similarities for researchers/scientists selecting their stain best suiting their specific needs.
Explanation of DAPI
DAPI (4′,6-diamidino-2-phenylindole) is a fluorescent stain that binds strongly to regions of DNA with AT bases. As such, this blue fluorescent dye is commonly used in molecular biology and cytogenetics to stain cell nuclei.
DAPI can be used to visually observe nuclear structures and chromosomal material within cells when bound to DNA, providing an excellent way of doing so. When excited at 358nm, its fluorescence occurs at approximately 461 nm in wavelength.
DAPI staining is widely employed for numerous applications, including examination of cell cycle progression, apoptosis, and chromosomal organization. Due to its specificity for DNA and ease of use, many researchers utilize it in various fields.
Its ability to fluoresce upon binding with DNA allows quantification and analysis of DNA within various cellular environments.
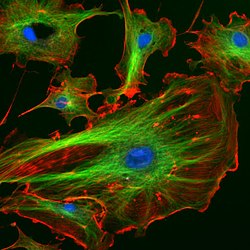
Explanation of Hoechst
Hoechst stains are fluorescent compounds used to stain DNA in cell biology. Common variants include Hoechst 33342 and Hoechst 33258, two blue fluorescent dyes commonly employed to mark nuclear structures and chromosomes with fluorescent labels.
Hoechst stains adhere to DNA’s minor groove, particularly at areas containing long stretches of adenine-thymine (AT) base pairs. When bound, UV light excites them and releases fluorescence-emitting particles which allow researchers to observe and analyze cell nuclei.
Hoechst 33342 and Hoechst 33258 differ slightly in their chemical structures and binding properties but are both used in similar applications such as studying cell cycle progression, apoptosis, chromosome organization, and organelles such as lymph nodes.
Their excitation/emission maxima typically vary between 361/486nm for Hoechst 33342 while with Hoechst 33258 these typically range between 352/461 nm.
Hoechst stains are esteemed for their strong affinity to DNA and compatibility with other cellular stains and markers, making them effective tools in cell biology research and experimentation.
Comparison Table of DAPI and Hoechst
Certainly! Below is a comparison table that outlines the key differences and similarities between DAPI and Hoechst stains:
| Feature | DAPI | Hoechst (e.g., 33342 & 33258) |
|---|---|---|
| Chemical Structure | 4′,6-diamidino-2-phenylindole | Different variants have slight variations |
| Color of Fluorescence | Blue | Blue |
| Excitation Wavelength | ~358 nm | 33342: ~361 nm, 33258: ~352 nm |
| Emission Wavelength | ~461 nm | 33342: ~486 nm, 33258: ~461 nm |
| Binding Site on DNA | AT-rich regions | The minor groove of AT-rich regions |
| Common Applications | Cell cycle analysis, apoptosis | Cell cycle analysis, apoptosis, chromosome visualization |
| Sensitivity | Moderate to high | High, depending on the variant |
| Compatibility with Other Stains | Good, but may vary | Often better due to different spectral properties |
| Cost | Generally cheaper | Typically more expensive |
This table summarizes the key aspects of DAPI and Hoechst, illustrating their similarities in usage and differences in properties like excitation/emission wavelengths and sensitivity. It’s essential to consider these factors when selecting the appropriate stain for a specific application or experiment.
Compatibility with Other Stains and Markers
Compatible interactions between DAPI and Hoechst with other stains and markers are critical for researchers performing multi-color fluorescence microscopy or complex staining procedures, such as complex staining procedures.
Here is an overview of their compatibility:
1. Compatible with Green and Red Fluorescent Markers: DAPI can be used alongside green (e.g., FITC) and red (e.g., TRITC, Texas Red) fluorescent markers without significant overlap, allowing simultaneous visualization of different cellular components.
2. Interaction With Antifade Mountants: DAPI Can Be Used alongside Green (FITC, Texas Red) and Red Fluorescent Markers Without Significant Overlap.
3. Antifade Mountant Interactions: Antifade mountants may reduce fluorescence caused by DAPI fluorescence; to maximize fluorescence it’s essential to select compatible mountants or apply it post mounting.
4. Hoechst 1 Broader Compatibility With Fluorescent Markers: Hoechst stains are often considered more compatible with other fluorescent markers due to their distinct spectral properties, making them suitable for live cell imaging.
5. Suited for Live Cell Imaging: Hoechst 33342 fluorescent dye is frequently employed for live cell imaging with other fluorescent markers to gain real-time insight into cellular processes.
6. Reduced Interaction With Antifade Mountants: Hoechst tends to be less susceptible to quenching by antifade mountants, making it simpler for use with other staining methods such as immunostaining. 4Works Well with Immunostaining
Hoechst stains can be seamlessly integrated with various immunostaining protocols, making them highly adaptable in multi-color staining applications.
As stated previously, both DAPI and Hoechst can be combined with various stains and markers, though their compatibility will depend on your specific experiment’s requirements and conditions.
Therefore, considering both properties as well as consulting relevant protocols and literature are key steps towards designing successful staining procedures.
Recommended Protocols and Concentrations
DAPI and Hoechst staining recommendations may depend on several factors, including application type, cell/tissue type being studied, and desired results.
Below are general guidelines that can be adjusted accordingly:
Concentration of Dapsone for Intraoperative Imaging: At present, 0.1-2ug/mL concentration is typically used.
With respect to the protocol:
Fix cells or tissues using an appropriate fixative (e.g., 4% paraformaldehyde). Wash with PBS or another buffer solution to remove excess fixative. Incubate with DAPI solution for 5-10 minutes before washing again to remove unbound DAPI molecules. Ultimately mount your work using an antifade mountant as necessary.
Visualize under a fluorescent microscope using UV excitation. Hoechst 33342 should typically be used at concentrations between 1-10 ug/mL. Hoechst 33258 can be applied at concentrations ranging from 0.5-5 ug/mL.
For live cells, use 15-30 minutes at 37degC for Hoechst stain incubation time at 37degC before fixing with DAPI fixative and washing steps as directed for fixed cells (similarly described for Hoechst). Clean the surface to remove any extra stain if necessary before washing to remove excess stain if necessary.
Continue with additional staining or mounting as necessary, using UV excitation. visualize under a fluorescence microscope using appropriate UV excitation. Note: For specific cell types and applications, concentration and incubation times should be tailored accordingly.
Hoechst stain must be used with great caution in live cell imaging as its concentration and incubation time must be carefully controlled to minimize potential toxicity. When handling this substance, precautions such as using appropriate personal protective equipment must also be observed.
Starting points such as these protocols and concentrations serve as starting points, with further customization depending on experimental conditions, cell types, or specific research questions. Consultation of product datasheets, relevant literature, and institutional guidelines may also prove helpful for tailor-made protocols.
Guidance on selecting the appropriate stain for specific needs
The selection of an appropriate stain between DAPI and Hoechst depends on a range of factors related to an experiment’s goals, specific requirements, and available resources.
Here are some guidelines to assist in making an informed choice:
1. Spectral Properties: For experiments involving other fluorescent markers within the green or red spectrum, DAPI may be suitable. Hoechst may provide greater compatibility if your experiment will include multiple fluorescent markers.
2. Sensitivity: Depending on the parameters you choose for the experiment, your probe might have different sensitivities to other markers; these should all be considered before selecting one as your detection antibody.
DAPI: Appropriate for most applications where high sensitivity isn’t essential.
Hoechst: Ideal for live cell imaging applications where higher sensitivity and specificity are key considerations. Hoechst is more expensive but should still provide reliable imaging results.
3 Cost Considerations: Hoechst may be preferable if cost is not your primary consideration and specific properties offered by Hoechst may be required for staining purposes.
4. Live Cells Vs Fixed Cells: DAPI should only be used on fixed cells when staining routine samples or when budget constraints exist, making it the more economical choice. 6.55% | Hoechst | Hoechst stain, particularly Hoechst 33342, should be considered for live cell staining.
5. Compatibility With Other Stains and Protocols: DAPI may need to be modified accordingly when used with specific antifade mountants that could interfere with its effects; carefully consider and modify your protocol as necessary.
Hoechst: For broad compatibility requirements, Hoechst may be more suitable.
6. Health and Safety Considerations: Both DAPI and Hoechst are potency mutagens that must be handled with great caution – make sure your laboratory has proper safety protocols in place as well as adequate facilities available to manage them safely.
7. Experimental Context: Examine the overall context of your experiment, such as its purpose, the types of samples involved, and any specific imaging or analysis needs.
Reference Books
- “Molecular Biology of the Cell” by Bruce Alberts et al.
- A comprehensive guide to cell biology, including details on fluorescent staining techniques.
- “Cell and Molecular Biology: Concepts and Experiments” by Gerald Karp
- Offers insights into molecular techniques, including cell staining and microscopy.
- “Methods in Cell Biology: Fluorescence Microscopy” (Various Volumes)
- A series focused on fluorescence microscopy methods, including the use of DAPI and Hoechst.
- “Handbook of Biological Confocal Microscopy” by James Pawley
- Details on various imaging techniques, including the utilization of fluorescent dyes.
- “Live Cell Imaging: A Laboratory Manual” by Robert D. Goldman and David L. Spector
- Focused on live cell imaging techniques, including the use of Hoechst for live cells.
Conclusion
DAPI and Hoechst fluorescent stains are essential fluorescent staining agents in cell biology, each offering unique characteristics and applications. While DAPI is typically chosen due to its cost-effectiveness and compatibility with fixed cells, Hoechst offers greater sensitivity for live cell imaging applications.
When choosing between them, key considerations include their spectral properties, sensitivity levels, cost considerations, experiment context as well as future developments which could offer new alternatives or enhanced methods expanding.